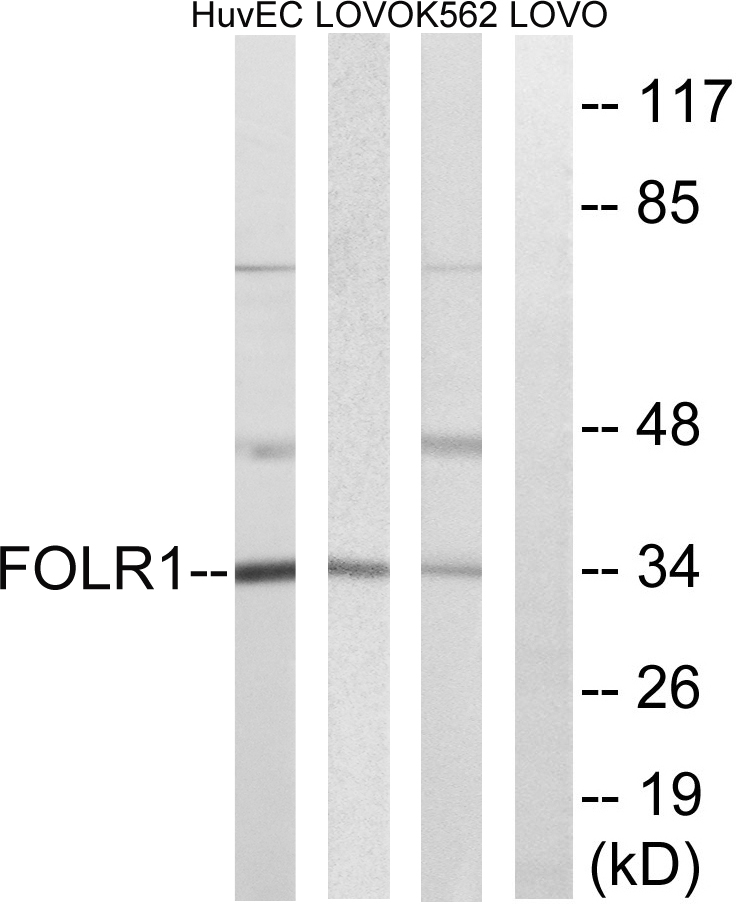
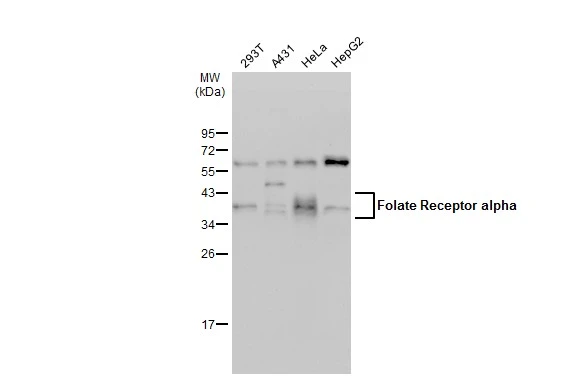
Anti-FOLR1 [Farletuzumab (MORAb-003; M3)]
Ab03043-3.3
ApplicationsFunctional Assay, ELISA, ImmunoHistoChemistry, Neutralisation/Blocking, Other Application
Product group Antibodies
ReactivityHuman, Monkey
TargetFOLR1
Overview
- SupplierAbsolute Antibody
- Product NameAnti-FOLR1 [Farletuzumab (MORAb-003; M3)]
- Delivery Days Customer7
- Application Supplier NoteThis antibody was derived from the optimization of mouse parental LK26 antibody using a whole-cell genetic evolution platform. This antibody possesses growth-inhibitory activity on cells overexpressing FR-alpha. It elicited robust antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC) in vitro and inhibited the growth of human ovarian tumor xenografts in nude mice. Immunohistochemistry studies determined that MORAb-003 infrequently stained the tubular epithelium, epithelium of the fallopian tube, and duct epithelium of the pancreas in both normal human and cynomolgus monkey tissues. Cynomolgus monkey was therefore considered an appropriate toxicology model (Ebel et al., 2007; PMID: 17346028). This antibody was also found to antagonize the activity of human FRalpha, resulting in the loss of growth advantage conferred by overexpression of FRalpha under conditions that bracket physiological (10-100 nM) folate concentrations (Routhier et al., 2006; DOI: 10.1200/jco.2006.24.18_suppl.10108). In a human ovarian cancer model, female athymic nude mice implanted with IGROV cells (expressing FRalpha) were treated with this antibody, Paclitaxel, or a combination of both. This antibody alone reduced tumor growth by approximately 30%, while Paclitaxel alone reduced tumor burden by about 65%. However, the combination of both resulted in greater than 80% growth inhibition (Maddage et al., 2012; DOI: 10.1158/1538-7445.AM2012-4411). A phase I study in patients with platinum-resistant ovarian cancer revealed that MORAb-003 was well tolerated in patients with epithelial ovarian cancers and may have activity in platinum-resistant patients (Konner et al., 2006; DOI: 10.1200/jco.2006.24.18_suppl.5027) (Bell-McGuinn et al., 2007; DOI: 10.1200/jco.2007.25.18_suppl.5553). In a phase 2 trial in patients with platinum-sensitive ovarian cancer, patients received single-agent farletuzumab or farletuzumab combined with carboplatin and paclitaxel (or docetaxel), followed by farletuzumab maintenance until progression. Of the 47 patients who received farletuzumab, 80.9% had normalization of their CA125, and a complete or partial objective response rate was achieved in 75% with combination therapy (Herzog et al., 2016; DOI: 10.1200/JCO.2016.34.15_suppl.TPS5608). It was also reported that farletuzumab with carboplatin and taxane may enhance the response rate and duration of response in platinum-sensitive ovarian cancer patients with first relapse after remission of 6-18 months (Armstrong et al., 2013; PMID: 23474348).
- ApplicationsFunctional Assay, ELISA, ImmunoHistoChemistry, Neutralisation/Blocking, Other Application
- Applications Supplierantagonist; ELISA; IHC; functional assay
- CertificationResearch Use Only
- ClonalityMonoclonal
- Clone IDFarletuzumab (MORAb-003; M3)
- Gene ID2348
- Target nameFOLR1
- Target descriptionfolate receptor alpha
- Target synonymsFBP, FOLR, FRalpha, NCFTD, folate receptor alpha, FR-alpha, KB cells FBP, adult folate-binding protein, folate binding protein, folate receptor 1 (adult), folate receptor, adult, ovarian tumor-associated antigen MOv18
- HostMouse
- IsotypeIgG2b
- Protein IDP15328
- Protein NameFolate receptor alpha
- Scientific DescriptionThis chimeric mouse antibody was made using the variable domain sequences of the original Human IgG1 format for improved compatibility with existing reagents assays and techniques.
- ReactivityHuman, Monkey
- Reactivity SupplierHuman, cynomolgus monkey
- Reactivity Supplier NoteThe original parental mouse antibody was generated by immunizing (BALB/c X C57BL/6) F1 mice by intraperitoneal injection containing cultured LU-75(c) choriocarcinoma cells to produce hybridomas that generated the antibody LK26. Later on the Farletuzumab was generated by CDR grafting technique by taking CDRs of mouse parental clone LK26 and grafting them on human framework regions.
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC41116161