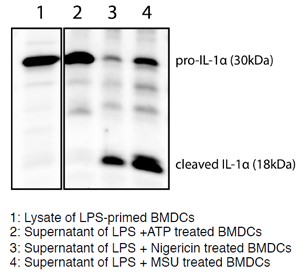
Mouse IL-1alpha (full-length p30 and cleaved p18 fragments) are detected by immunoblotting using anti-IL-1alpha (p18) (mouse), mAb (Teo-1) (Prod. No AG-20B-0064). Method: IL-1alpha was analyzed by Western blot in cell extracts of bone m
anti-IL-1alpha (p18) (mouse), mAb (Teo-1)
AG-20B-0064
ApplicationsWestern Blot, ELISA
Product group Antibodies
ReactivityMouse
TargetIl1a
Overview
- SupplierAdipoGen Life Sciences
- Product Nameanti-IL-1alpha (p18) (mouse), mAb (Teo-1)
- Delivery Days Customer10
- ApplicationsWestern Blot, ELISA
- CertificationResearch Use Only
- ClonalityMonoclonal
- Clone IDTeo-1
- Concentration1 mg/ml
- Estimated Purity>95%
- Gene ID16175
- Target nameIl1a
- Target descriptioninterleukin 1 alpha
- Target synonymsIl-1a, interleukin-1 alpha, IL-1 alpha
- HostMouse
- IsotypeIgG
- Protein IDP01582
- Protein NameInterleukin-1 alpha
- Scientific DescriptionMonoclonal Antibody. Recognizes mouse IL-1alpha p18 cleaved and full-length fragments. Isotype: Mouse IgG. Clone: Teo-1. Applications: ELISA, WB. Liquid. In PBS containing 10% glycerol and 0.02% sodium azide. The most prominent members of the interleukin-1 (IL-1) superfamily are IL-1alpha and IL-1beta. They lack a signal peptide and are secreted by an unconventional, endoplasmic reticulum-Golgi-independent mechanism. IL-1alpha was reported to be more widely and constitutively expressed and has intracellular functions, but also acts locally in a membrane-bound form by activating IL-1R1. Additionally, passive release of IL-1alpha upon cell death can trigger a sterile inflammatory response to dying cells. The cleavage of IL-1alpha is not mediated by caspase-1 and is not required for binding to IL-1R1. Recently it has been observed that all activators of the inflammasome NLRP3/NALP3 induce the simultaneous secretion of IL-1alpha and IL-1beta. Although most activators fully rely on the inflammasome for IL-1alpha secretion, some induce the processing and secretion of IL-1alpha in an inflammasome-independent manner. - The most prominent members of the interleukin-1 (IL-1) superfamily are IL-1alpha and IL-1beta. They lack a signal peptide and are secreted by an unconventional, endoplasmic reticulum-Golgi-independent mechanism. IL-1alpha was reported to be more widely and constitutively expressed and has intracellular functions, but also acts locally in a membrane-bound form by activating IL-1R1. Additionally, passive release of IL-1alpha upon cell death can trigger a sterile inflammatory response to dying cells. The cleavage of IL-1alpha is not mediated by caspase-1 and is not required for binding to IL-1R1. Recently it has been observed that all activators of the inflammasome NLRP3/NALP3 induce the simultaneous secretion of IL-1alpha and IL-1beta. Although most activators fully rely on the inflammasome for IL-1alpha secretion, some induce the processing and secretion of IL-1alpha in an inflammasome-independent manner.
- ReactivityMouse
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC41116161





![Sandwich ELISA analysis of mouse IL-1alpha protein using GTX02969 IL1 alpha antibody [MT946] as coating antibody and GTX02970-02 IL1 alpha antibody [MT951] (Biotin) as detecting antibody.](https://www.genetex.com/upload/website/prouct_img/normal/GTX02969/GTX02969_20210507_ELISA_w_23053123_405.webp)