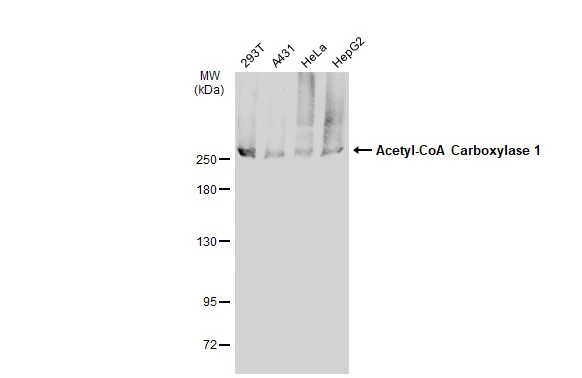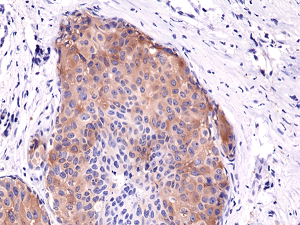
Immunohistochemical staining of formalin fixed and paraffin embedded human breast cancer tissue sections using Anti-Phospho-Acetyl CoA Carboxylase (Ser79) Rabbit Monoclonal Antibody (clone RM270) at a 1:5000 dilution.
anti-Phospho Acetyl CoA Carboxylase (S79) (human), Rabbit Monoclonal (RM270)
REV-31-1151-00
ApplicationsWestern Blot, ImmunoHistoChemistry
Product group Antibodies
ReactivityHuman
TargetACACA
Overview
- SupplierRevMAb Biosciences
- Product Nameanti-Phospho Acetyl CoA Carboxylase (S79) (human), Rabbit Monoclonal (RM270)
- Delivery Days Customer2
- ApplicationsWestern Blot, ImmunoHistoChemistry
- CertificationResearch Use Only
- ClonalityMonoclonal
- Clone IDRM270
- Gene ID31
- Target nameACACA
- Target descriptionacetyl-CoA carboxylase alpha
- Target synonymsACAC, ACACAD, ACACalpha, ACC, ACC1, ACCA, ACCalpha, Acac1, hACC1, acetyl-CoA carboxylase 1, ACC-alpha, acetyl-Coenzyme A carboxylase alpha
- HostRabbit
- IsotypeIgG
- Protein IDQ13085
- Protein NameAcetyl-CoA carboxylase 1
- Scientific DescriptionAcetyl-CoA carboxylase (ACC) is a biotin-dependent enzyme that catalyzes the irreversible carboxylation of acetyl-CoA to produce malonyl-CoA through its two catalytic activities, biotin carboxylase (BC) and carboxyltransferase (CT). The function of ACC is to regulate the metabolism of fatty acids. When the enzyme is active, the product, malonyl-CoA, is produced which is a building block for new fatty acids and can inhibit the transfer of the fatty acyl group from acyl CoA to carnitine with carnitine acyltransferase, which inhibits the beta-oxidation of fatty acids in the mitochondria. There are two ACC forms, alpha (ACC1) and beta (ACC2), encoded by two different genes. ACC-alpha is highly enriched in lipogenic tissues. The enzyme is under long term control at the transcriptional and translational levels and under short term regulation by the phosphorylation/dephosphorylation of targeted serine residues and by allosteric transformation by citrate or palmitoyl-CoA. Phosphorylation by AMPK at Ser79 or by PKA at Ser1200 inhibits the enzymatic activity of ACC. - Recombinant Antibody. This antibody reacts to human Acetyl CoA Carboxylase (ACC1) only when phosphorylated at Ser79. There is no cross-reactivity to Acetyl CoA Carboxylase without phosphorylation at Ser79. Applications: WB, IHC. Source: Rabbit. Liquid. 50% Glycerol/PBS with 1% BSA and 0.09% sodium azide. Acetyl-CoA carboxylase (ACC) is a biotin-dependent enzyme that catalyzes the irreversible carboxylation of acetyl-CoA to produce malonyl-CoA through its two catalytic activities, biotin carboxylase (BC) and carboxyltransferase (CT). The function of ACC is to regulate the metabolism of fatty acids. When the enzyme is active, the product, malonyl-CoA, is produced which is a building block for new fatty acids and can inhibit the transfer of the fatty acyl group from acyl CoA to carnitine with carnitine acyltransferase, which inhibits the beta-oxidation of fatty acids in the mitochondria. There are two ACC forms, alpha (ACC1) and beta (ACC2), encoded by two different genes. ACC-alpha is highly enriched in lipogenic tissues. The enzyme is under long term control at the transcriptional and translational levels and under short term regulation by the phosphorylation/dephosphorylation of targeted serine residues and by allosteric transformation by citrate or palmitoyl-CoA. Phosphorylation by AMPK at Ser79 or by PKA at Ser1200 inhibits the enzymatic activity of ACC.
- ReactivityHuman
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC41116161