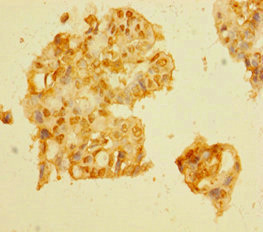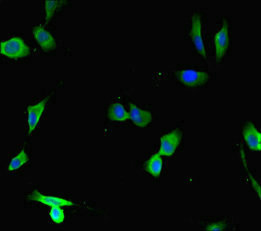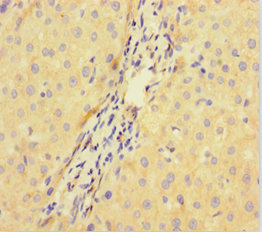
Immunohistochemistry of paraffin-embedded human liver cancer using CSB-PA896722LA01HU at dilution of 1:100
DCAF1 Antibody
CSB-PA896722LA01HU
ApplicationsImmunoFluorescence, ELISA, ImmunoHistoChemistry
Product group Antibodies
ReactivityHuman
TargetDCAF1
Overview
- SupplierCusabio
- Product NameDCAF1 Antibody
- Delivery Days Customer20
- ApplicationsImmunoFluorescence, ELISA, ImmunoHistoChemistry
- CertificationResearch Use Only
- ClonalityPolyclonal
- ConjugateUnconjugated
- Gene ID9730
- Target nameDCAF1
- Target descriptionDDB1 and CUL4 associated factor 1
- Target synonymsRIP, VPRBP, DDB1- and CUL4-associated factor 1, HIV-1 Vpr-binding protein, Vpr (HIV-1) binding protein, Vpr-binding protein, protein VPRBP, serine/threonine-protein kinase VPRBP, vpr-interacting protein
- HostRabbit
- IsotypeIgG
- Protein IDQ9Y4B6
- Protein NameDDB1- and CUL4-associated factor 1
- Scientific DescriptionActs both as a substrate recognition component of E3 ubiquitin-protein ligase complexes and as an atypical serine/threonine-protein kinase, playing key roles in various processes such as cell cycle, telomerase regulation and histone modification. Probable substrate-specific adapter of a DCX (DDB1-CUL4-X-box) E3 ubiquitin-protein ligase complex, named CUL4A-RBX1-DDB1-DCAF1/VPRBP complex, which mediates ubiquitination and proteasome-dependent degradation of proteins such as NF2. Involved in the turnover of methylated proteins: recognizes and binds methylated proteins via its chromo domain, leading to ubiquitination of target proteins by the RBX1-DDB1-DCAF1/VPRBP complex (PubMed:23063525). The CUL4A-RBX1-DDB1-DCAF1/VPRBP complex is also involved in B-cell development: VPRBP is recruited by RAG1 to ubiquitinate proteins, leading to limit error-prone repair during V(D)J recombination. Also part of the EDVP complex, an E3 ligase complex that mediates ubiquitination of proteins such as TERT, leading to TERT degradation and telomerase inhibition (PubMed:23362280). Also acts as an atypical serine/threonine-protein kinase that specifically mediates phosphorylation of Thr-120 of histone H2A (H2AT120ph) in a nucleosomal context, thereby repressing transcription. H2AT120ph is present in the regulatory region of many tumor suppresor genes, down-regulates their transcription and is present at high level in a number of tumors (PubMed:24140421). Involved in JNK-mediated apoptosis during cell competition process via its interaction with LLGL1 and LLGL2 (PubMed:20644714). In case of infection by HIV-1 virus, it is recruited by HIV-1 Vpr in order to hijack the CUL4A-RBX1-DDB1-DCAF1/VPRBP function leading to arrest the cell cycle in G2 phase, and also to protect the viral protein from proteasomal degradation by another E3 ubiquitin ligase. The HIV-1 Vpr protein hijacks the CUL4A-RBX1-DDB1-DCAF1/VPRBP complex to promote ubiquitination and degradation of proteins such as TERT and ZIP/ZGPAT. In case of infection by HIV-2 virus, it is recruited by HIV-2 Vpx in order to hijack the CUL4A-RBX1-DDB1-DCAF1/VPRBP function leading to enhanced efficiency of macrophage infection and promotion of the replication of cognate primate lentiviruses in cells of monocyte/macrophage lineage.
- ReactivityHuman
- Storage Instruction-20°C or -80°C
- UNSPSC41116161