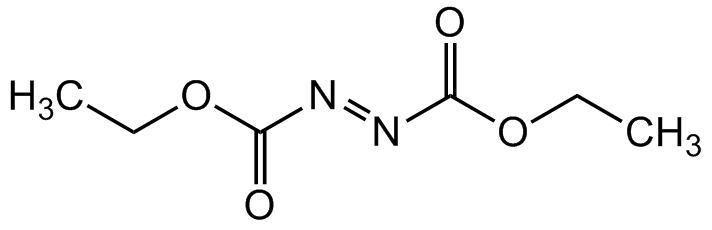
Chemical Structure
Diethyl azodicarboxylate [1972-28-7] [1972-28-7]
CDX-D0095
CAS Number1972-28-7
Product group Chemicals
Estimated Purity>98%
Molecular Weight174.15
Overview
- SupplierChemodex
- Product NameDiethyl azodicarboxylate [1972-28-7] [1972-28-7]
- Delivery Days Customer10
- CAS Number1972-28-7
- CertificationResearch Use Only
- Estimated Purity>98%
- Hazard InformationWarning
- Molecular FormulaC6H10N2O4
- Molecular Weight174.15
- Scientific DescriptionChemical. CAS: 1972-28-7. Formula: C6H10N2O4. MW: 174.15. Synthetic. Reagent for synthesis. Diethyl azodicarboxylate acts as a dienophile used in Diels-Alder reactions. It is used as a reagent in alpha-thiocyanation of enolizable ketones with ammonium thiocyanate and annulation of N-protected imines. It acts as a reactant for the preparation of immunostimulants alpha-Galactosylceramides, bisubstrate inhibitors with molecular recognition at the active site of catechol-O-methyltransferase and aza-beta-lactams via NHC-catalyzed [2 + 2] cycloaddition with ketenes. It is a commonly used activating reagent in Mitsunobu reaction and used in the synthesis of pharmaceuticals like zidovudine and procarbazine. In addition, it is an efficient dehydrogenating agent, which is involved in the preparation of aldehydes, disulfides, hydrazo groups from alcohols, thiols and azo goups, respectively. It is also a good electron acceptor. It is mostly known as a key component of the Mitsunobu reaction, a common strategy for the preparation of an amine, azide, ether, thioether or ester from the corresponding alcohol. - Reagent for synthesis. Diethyl azodicarboxylate acts as a dienophile used in Diels-Alder reactions. It is used as a reagent in alpha-thiocyanation of enolizable ketones with ammonium thiocyanate and annulation of N-protected imines. It acts as a reactant for the preparation of immunostimulants alpha-Galactosylceramides, bisubstrate inhibitors with molecular recognition at the active site of catechol-O-methyltransferase and aza-beta-lactams via NHC-catalyzed [2 + 2] cycloaddition with ketenes. It is a commonly used activating reagent in Mitsunobu reaction and used in the synthesis of pharmaceuticals like zidovudine and procarbazine. In addition, it is an efficient dehydrogenating agent, which is involved in the preparation of aldehydes, disulfides, hydrazo groups from alcohols, thiols and azo goups, respectively. It is also a good electron acceptor. It is mostly known as a key component of the Mitsunobu reaction, a common strategy for the preparation of an amine, azide, ether, thioether or ester from the corresponding alcohol.
- SMILESO=C(/N=N/C(OCC)=O)OCC
- Storage InstructionRT
- UNSPSC12352200