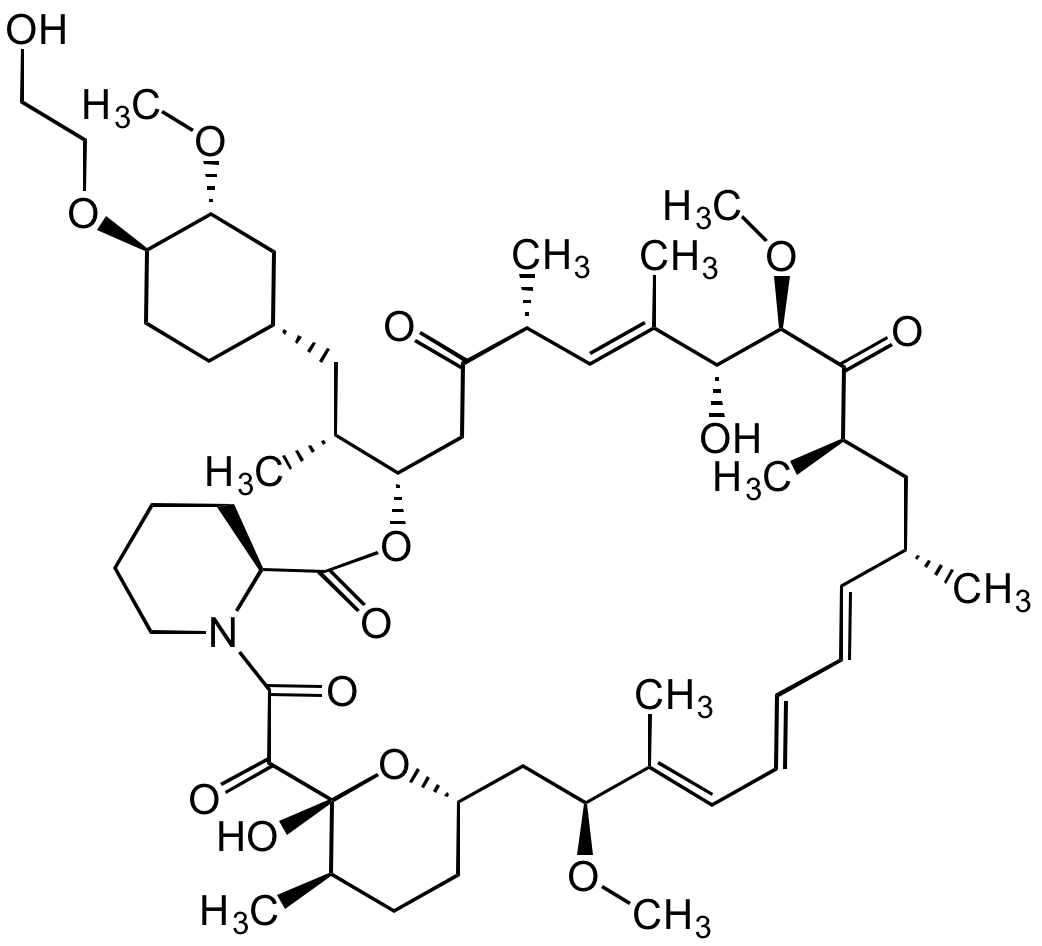
Chemical Structure
Everolimus [159351-69-6] [159351-69-6]
CDX-E0074
CAS Number159351-69-6
Product group Chemicals
Estimated Purity>99%
Molecular Weight958.2
Overview
- SupplierChemodex
- Product NameEverolimus [159351-69-6] [159351-69-6]
- Delivery Days Customer2
- CAS Number159351-69-6
- CertificationResearch Use Only
- Estimated Purity>99%
- Hazard InformationDanger
- Molecular FormulaC53H83NO14
- Molecular Weight958.2
- Scientific DescriptionChemical. CAS: 159351-69-6. Formula: C53H83NO14. MW: 958.2. Isolated from Streptomyces hygroscopicus. Macrolide antibiotic, inhibiting bacterial protein synthesis. Potent immunosuppressant. Binds with high affinity to the FK506 binding protein-12 (FKBP-12) to generate an immunosuppressive complex that inhibits the activation of the mammalian target of rapamycin (mTOR). More selective for the mTORC1 protein complex, with little impact on the mTORC2 complex, compared to Rapamycin. Anticancer agent. Inhibition of mTOR reduces the activity of effectors downstream, which leads to a blockage in the progression of cells from G1 into S phase, and subsequently inducing cell growth arrest, apoptosis and autophagy, resulting in reduction of cell proliferation, angiogenesis and glucose uptake. Inhibits tumor proliferation in vitro and in vivo. - Macrolide antibiotic, inhibiting bacterial protein synthesis. Potent immunosuppressant. Binds with high affinity to the FK506 binding protein-12 (FKBP-12) to generate an immunosuppressive complex that inhibits the activation of the mammalian target of rapamycin (mTOR). More selective for the mTORC1 protein complex, with little impact on the mTORC2 complex, compared to Rapamycin. Anticancer agent. Inhibition of mTOR reduces the activity of effectors downstream, which leads to a blockage in the progression of cells from G1 into S phase, and subsequently inducing cell growth arrest, apoptosis and autophagy, resulting in reduction of cell proliferation, angiogenesis and glucose uptake. Inhibits tumor proliferation in vitro and in vivo.
- SMILESOCCO[C@@H]1CC[C@@H](C[C@H]([C@@H]2CC([C@@H](/C=C([C@H]([C@H](C([C@@H](C[C@@H](/C=C/C=C/C=C([C@H](C[C@@H]3CC[C@H]([C@@](O3)(C(C(N4CCCC[C@H]4C(O2)=O)=O)=O)O)C)OC)\C)C)C)=O)OC)O)\C)C)=O)C)C[C@H]1OC
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200

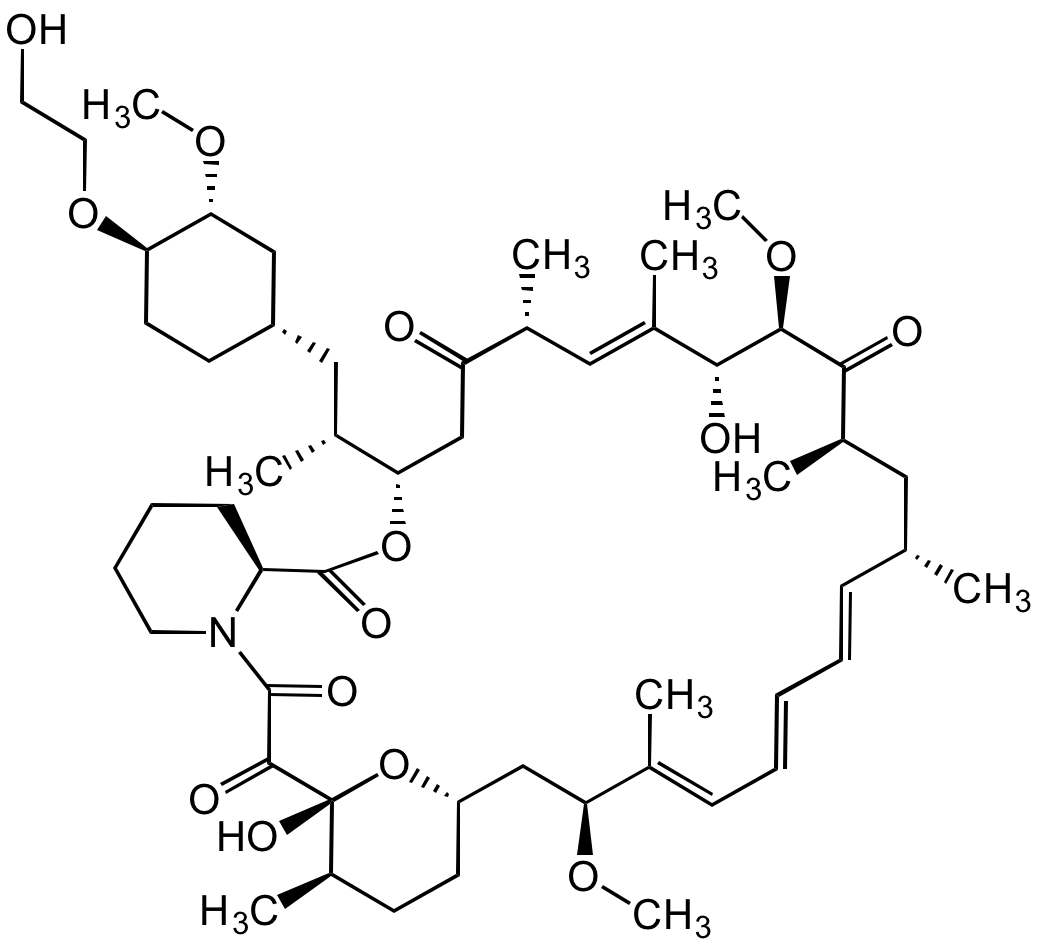
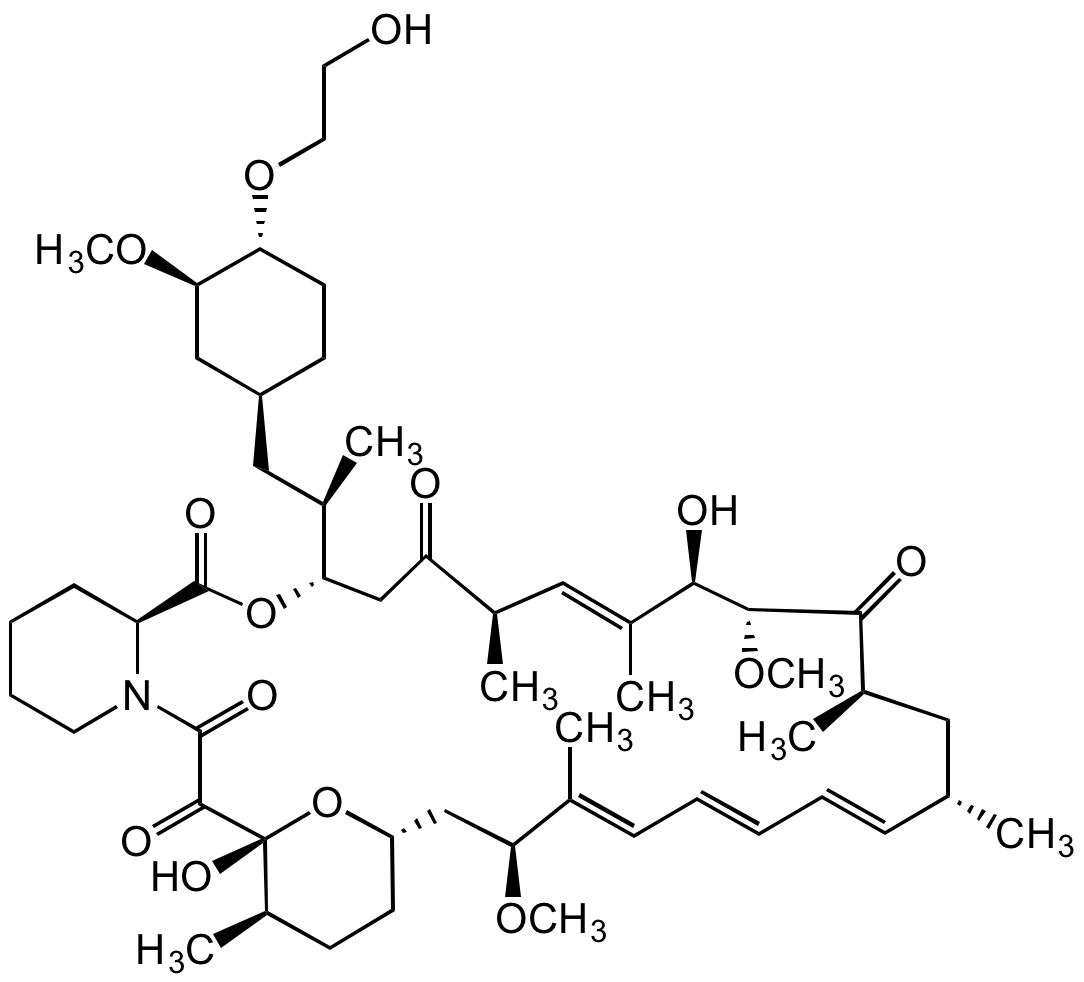

![Everolimus [159351-69-6]](https://www.targetmol.com/group3/M00/37/33/CgoaEGaySW6EfO-MAAAAAO061BM886.png)