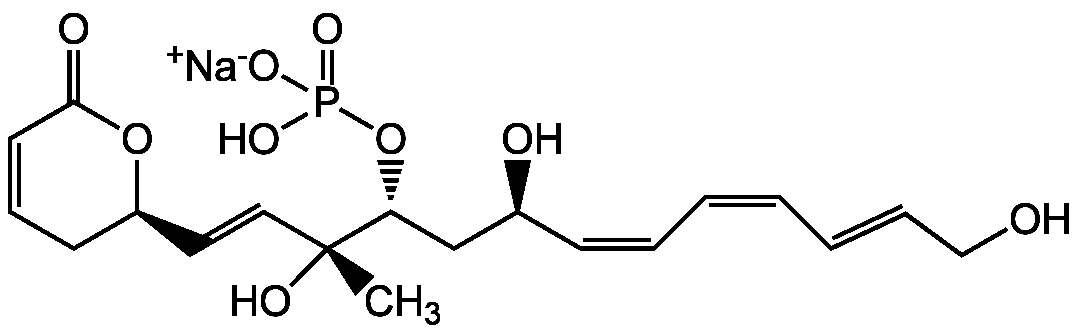
Chemical Structure
Fostriecin [87860-39-7] [87860-39-7]
AG-CN2-0057
CAS Number87860-39-7
Product group Chemicals
Estimated Purity>95%
Molecular Weight452.4
Overview
- SupplierAdipoGen Life Sciences
- Product NameFostriecin [87860-39-7] [87860-39-7]
- Delivery Days Customer10
- CAS Number87860-39-7
- CertificationResearch Use Only
- Estimated Purity>95%
- Hazard InformationWarning
- Molecular FormulaC19H26O9PNa
- Molecular Weight452.4
- Scientific DescriptionAntibiotic [1, 2, 18]. Anticancer compound [1-5, 8, 9, 11]. Antifungal [6]. Catalytic inhibitor of topoisomerase II (IC50 = 40 microM) [7, 16]. Potent protein phosphatase 2A (PP2A) (IC50 = 1.5 nM) and 4 (PP4) (IC50 = 3 nM) inhibitor [10, 12, 15]. Weak protein phosphatase type 1 (PP1) inhibitor (IC50=131microM). No apparent inhibition on PP2B [10, 12]. Mitotic entry checkpoint inhibitor [10]. Mediates cell cycle arrest at G2-M-phase [14]. Ischemia protective [13]. The PP2A binding site is different from that of okadaic acid [17, 19] - Chemical. CAS: 87860-39-7. Formula: C19H26O9PNa. MW: 452.4. Isolated from Streptomyces pulveraceous subsp. fostreus. Antibiotic. Anticancer compound. Antifungal. Catalytic inhibitor of topoisomerase II (IC50 = 40 microM). Potent protein phosphatase 2A (PP2A) (IC50 = 1.5 nM) and 4 (PP4) (IC50 = 3 nM) inhibitor. Weak protein phosphatase type 1 (PP1) inhibitor (IC50=131microM). No apparent inhibition on PP2B. Mitotic entry checkpoint inhibitor. Mediates cell cycle arrest at G2-M-phase. Ischemia protective. The PP2A binding site is different from that of okadaic acid
- SMILES[Na+].C[C@@](O)(\C=C\[C@H]1CC=CC(=O)O1)[C@@H](C[C@@H](O)\C=C/C=C\C=C\CO)OP(O)([O-])=O
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200
