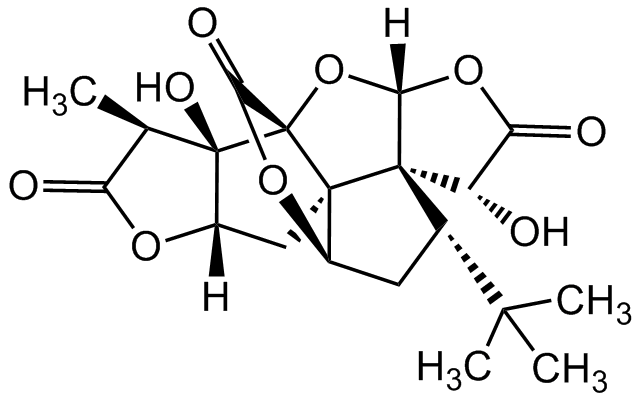
Chemical Structure
Ginkgolide A
CDX-G0221
CAS Number15291-75-5
Product group Chemicals
Estimated Purity>98%
Molecular Weight408.4
Overview
- SupplierChemodex
- Product NameGinkgolide A
- Delivery Days Customer10
- CAS Number15291-75-5
- CertificationResearch Use Only
- Estimated Purity>98%
- Molecular FormulaC20H24O9
- Molecular Weight408.4
- Scientific DescriptionChemical. CAS: 15291-75-5. Formula: C20H24O9. MW: 408.40. Ginkgolide A is a terpenoid lactone originally isolated from G. biloba leaves. It shows diverse biological activities, including antioxidant, neuroprotective, anti-inflammatory and antiproliferative effects. Ginkgolide A inhibits platelet activating factor (PAF)-dependent aggregation of human platelets. It inhibits nitric oxide (NO) production. It also shows anxiolytic-like activity. It Inhibits NF-kappa activity by NF-kappa-specific suppressor IkappaBalpha. Ginkgolide A is a ligand of pregnane X receptor (PXR). It showed hepatoprotective efficacy by inducing cellular lipoapoptosis and by inhibiting cellular inflammation in NAFLD. Ginkgolide A reduced the proliferation rate of OVCA429 ovarian cancer cells by 40%. - Ginkgolide A is a terpenoid lactone originally isolated from G. biloba leaves. It shows diverse biological activities, including antioxidant, neuroprotective, anti-inflammatory and antiproliferative effects. Ginkgolide A inhibits platelet activating factor (PAF)-dependent aggregation of human platelets. It inhibits nitric oxide (NO) production. It also shows anxiolytic-like activity. It Inhibits NF-kappa activity by NF-kappa-specific suppressor IkappaBalpha. Ginkgolide A is a ligand of pregnane X receptor (PXR). It showed hepatoprotective efficacy by inducing cellular lipoapoptosis and by inhibiting cellular inflammation in NAFLD. Ginkgolide A reduced the proliferation rate of OVCA429 ovarian cancer cells by 40%.
- SMILESO=C1[C@@H](C)[C@]([C@](O1)([H])C2)(O)[C@]([C@]32[C@H]4C[C@@H](C(C)(C)C)[C@]53[C@H]6O)(C(O4)=O)O[C@]5([H])OC6=O
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200

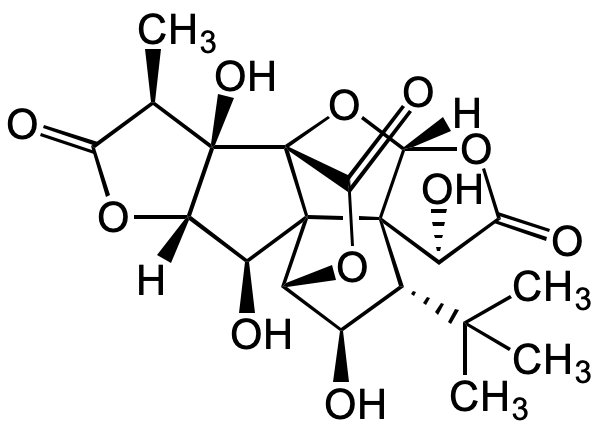

![Ginkgolide A [15291-75-5]](https://www.targetmol.com/group3/M00/35/43/CgoaEGayGjyEFKfYAAAAAEoAuO0310.png)