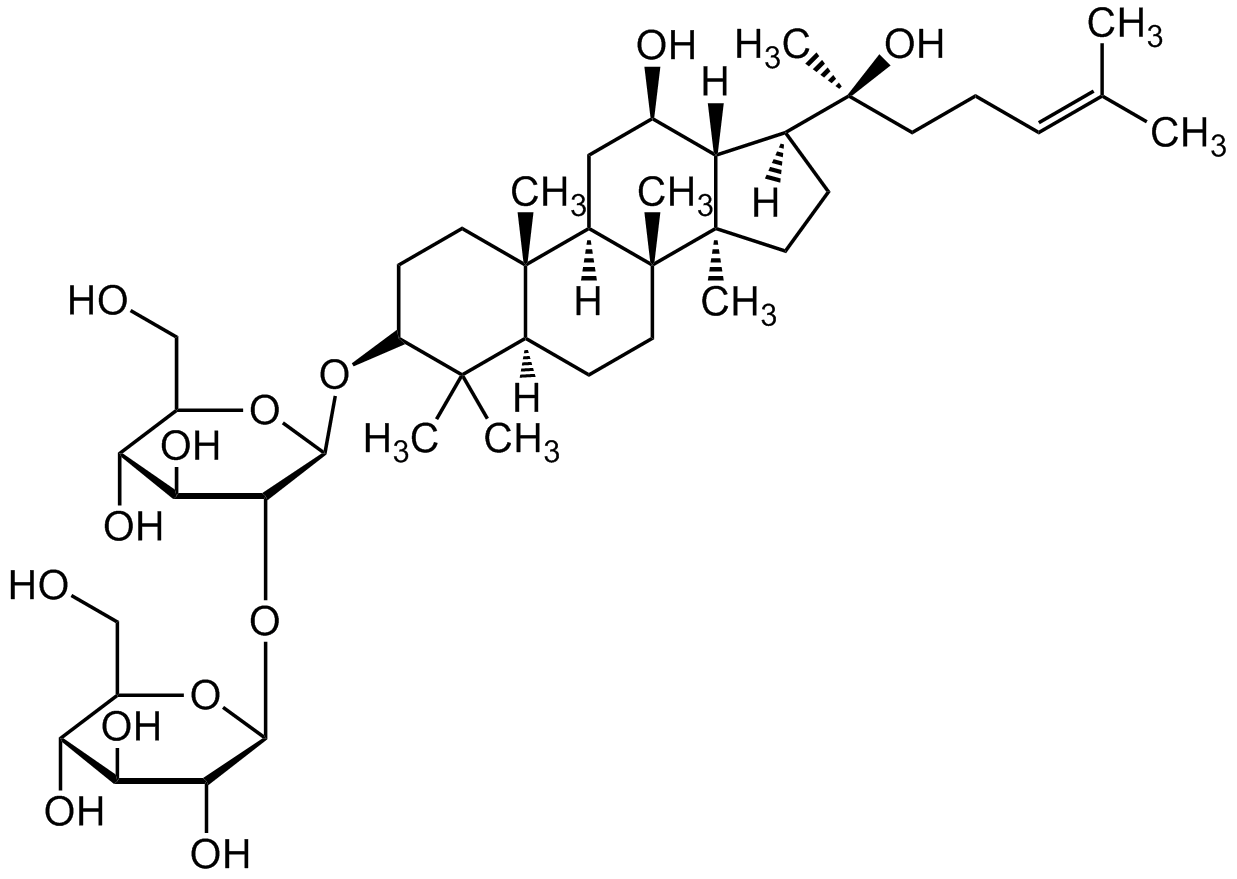
Chemical Structure
Ginsenoside Rg3 [14197-60-5] [14197-60-5]
CDX-G0220
CAS Number14197-60-5
Product group Chemicals
Estimated Purity>98%
Molecular Weight785.01
Overview
- SupplierChemodex
- Product NameGinsenoside Rg3 [14197-60-5] [14197-60-5]
- Delivery Days Customer2
- CAS Number14197-60-5
- CertificationResearch Use Only
- Estimated Purity>98%
- Molecular FormulaC42H72O13
- Molecular Weight785.01
- Scientific DescriptionChemical. CAS: 14197-60-5. Formula: C42H72O13. MW: 785.01. Ginsenoside Rg3 is a panaxadiol found in white and red P. ginseng. Show a broad range of biological in vitro and in vivo effects, including anticancer, antidiabetic, neuroprotective, antioxidant, anti-hypertensive, and anti-inflammatory actions. The anticancer mechanisms include induction of apoptosis and autophagy, inhibition of proliferation, inhibition of metastasis and angiogenesis, cell cycle arrest, immunomodulatory effects, sensitization to radiation, reducing multidrug resistance and inducing genotoxicity to the cancer cells. Ginsenoside Rg3 has been shown to inhibit the 5-HT3A and alpha3beta4 nACh receptors, the voltage-dependent Ca2+, K+, and Na+ channel currents. It is a scavenger of hydroxyl radicals and downregulated the expression of DNA methyltransferases, reducing global DNA methylation, modifying the methylation of the promoter region of some relevant genes in cancer. It enhances glucose-stimulated insulin secretion and activates AMPK. Ginsenoside Rg3 regulates NF-kappaB activity and suppresses the NLRP3 inflammasome activation through inhibition of its assembly. - Ginsenoside Rg3 is a panaxadiol found in white and red P. ginseng. Show a broad range of biological in vitro and in vivo effects, including anticancer, antidiabetic, neuroprotective, antioxidant, anti-hypertensive, and anti-inflammatory actions. The anticancer mechanisms include induction of apoptosis and autophagy, inhibition of proliferation, inhibition of metastasis and angiogenesis, cell cycle arrest, immunomodulatory effects, sensitization to radiation, reducing multidrug resistance and inducing genotoxicity to the cancer cells. Ginsenoside Rg3 has been shown to inhibit the 5-HT3A and alpha3beta4 nACh receptors, the voltage-dependent Ca2+, K+, and Na+ channel currents. It is a scavenger of hydroxyl radicals and downregulated the expression of DNA methyltransferases, reducing global DNA methylation, modifying the methylation of the promoter region of some relevant genes in cancer. It enhances glucose-stimulated insulin secretion and activates AMPK. Ginsenoside Rg3 regulates NF-kappaB activity and suppresses the NLRP3 inflammasome activation through inhibition of its assembly.
- SMILESOC[C@@H]1[C@@H](O)[C@H](O)[C@@H](O)[C@H](O[C@@H]2[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]2O[C@H]3C(C)(C)[C@@](CC[C@]4(C)[C@]5([H])C[C@@H](O)[C@@]6([H])[C@]4(CC[C@@]6([C@@](C)(O)CC/C=C(C)/C)[H])C)([H])[C@]5(C)CC3)O1
- Storage Instruction2°C to 8°C,RT
- UNSPSC12352200

![20(S)-Ginsenoside Rg3 [14197-60-5] [14197-60-5]](https://www.targetmol.com/group3/M00/35/67/CgoaEGayHkuEN0nVAAAAAHE0n1M759.png)