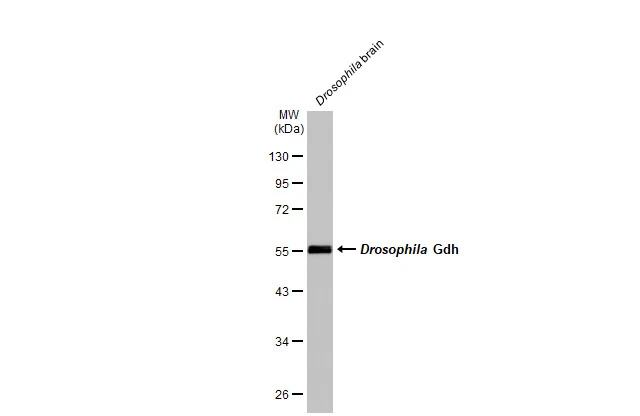
Drosophila tissue extract (50 μg) was separated by 10% SDS-PAGE, and the membrane was blotted with GLUD1 + GLUD2 antibody (GTX105765) diluted at 1:1000. The HRP-conjugated anti-rabbit IgG antibody (GTX213110-01) was used to detect the primary antibody.
GLUD1 + GLUD2 antibody
GTX105765
ApplicationsImmunoFluorescence, Western Blot, ELISA, ImmunoCytoChemistry, ImmunoHistoChemistry, ImmunoHistoChemistry Paraffin
Product group Antibodies
ReactivityDrosophila, Human, Mouse, Rat
TargetGLUD1
Overview
- SupplierGeneTex
- Product NameGLUD1 + GLUD2 antibody
- Delivery Days Customer9
- Application Supplier NoteWB: 1:500-1:3000. ICC/IF: 1:100-1:1000. IHC-P: 1:100-1:1000. *Optimal dilutions/concentrations should be determined by the researcher.Not tested in other applications.
- ApplicationsImmunoFluorescence, Western Blot, ELISA, ImmunoCytoChemistry, ImmunoHistoChemistry, ImmunoHistoChemistry Paraffin
- CertificationResearch Use Only
- ClonalityPolyclonal
- Concentration0.07 mg/ml
- ConjugateUnconjugated
- Gene ID2746
- Target nameGLUD1
- Target descriptionglutamate dehydrogenase 1
- Target synonymsGDH, GDH1, GLUD, hGDH1, glutamate dehydrogenase 1, mitochondrial, epididymis secretory sperm binding protein, epididymis tissue sperm binding protein Li 18mP, glutamate dehydrogenase (NAD(P)+)
- HostRabbit
- IsotypeIgG
- Protein IDP00367
- Protein NameGlutamate dehydrogenase 1, mitochondrial
- Scientific DescriptionL-glutamate dehydrogenase (EC 1.4.1.3) has a central role in nitrogen metabolism in plants and animals. Glutamate dehydrogenase is found in all organisms and catalyzes the oxidative deamination of 1-glutamate to 2-oxoglutarate (Smith et al., 2001 [PubMed 11254391]). Glutamate, the main substrate of GLUD, is present in brain in concentrations higher than in other organs. In nervous tissue, GLUD appears to function in both the synthesis and the catabolism of glutamate and perhaps in ammonia detoxification (Mavrothalassitis et al., 1988 [PubMed 3368458]).[supplied by OMIM]
- ReactivityDrosophila, Human, Mouse, Rat
- Storage Instruction-20°C or -80°C,2°C to 8°C
- UNSPSC41116161

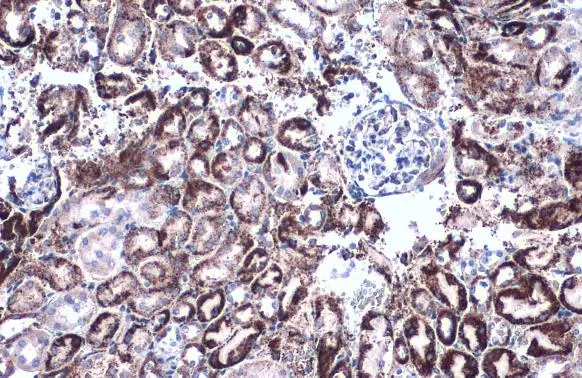
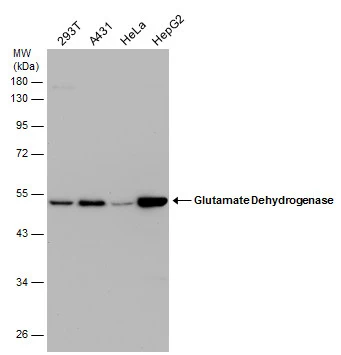
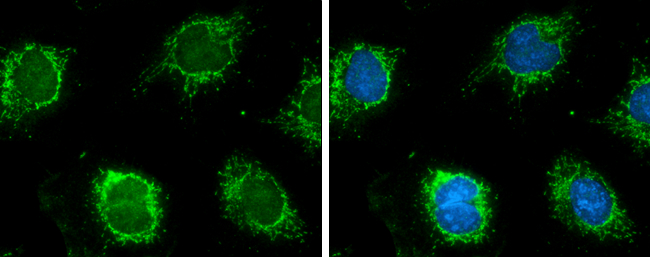
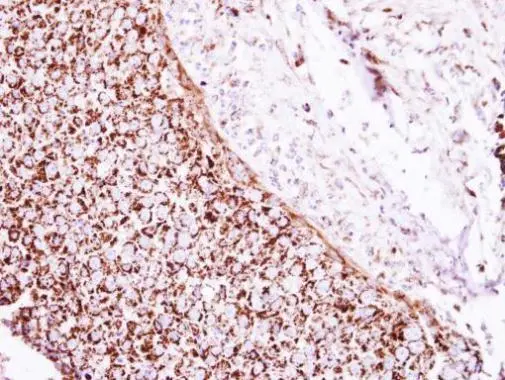
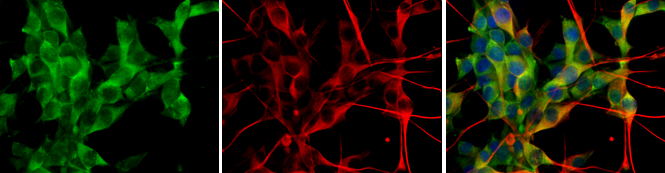
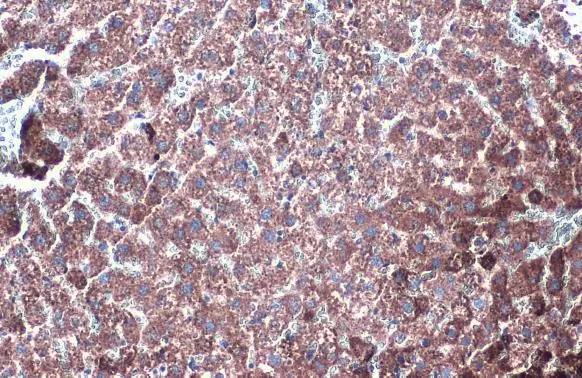
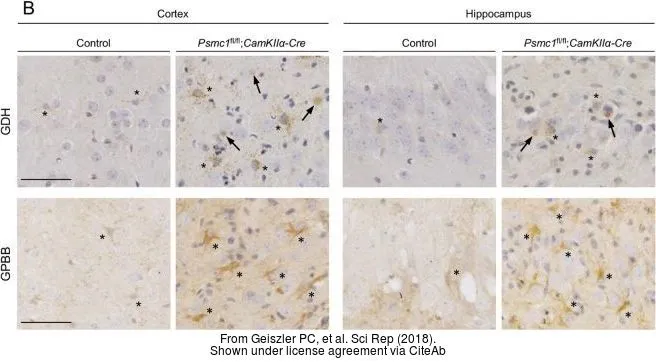





![Various tissue extracts (50 μg) were separated by 7.5% SDS-PAGE, and the membrane was blotted with GLUD1+GLUD2 antibody [HL2124] (GTX638096) diluted at 1:10000. The HRP-conjugated anti-rabbit IgG antibody (GTX213110-01) was used to detect the primary antibody.](https://www.genetex.com/upload/website/prouct_img/normal/GTX638096/GTX638096_T-44914_20230203_WB_M_R_23020621_380.webp)