![Immunoprecipitation of GRP94 protein from HeLa whole cell extracts using 5 μg of GRP94 antibody [N1N3] (GTX103203). Western blot analysis was performed using GRP94 antibody [N1N3] (GTX103203). EasyBlot anti-Rabbit IgG (GTX221666-01) was used as a secondary reagent. Immunoprecipitation of GRP94 protein from HeLa whole cell extracts using 5 μg of GRP94 antibody [N1N3] (GTX103203). Western blot analysis was performed using GRP94 antibody [N1N3] (GTX103203). EasyBlot anti-Rabbit IgG (GTX221666-01) was used as a secondary reagent.](https://www.genetex.com/upload/website/prouct_img/normal/GTX103203/GTX103203_40016_20150522_IP_w_23060119_571.webp)
Immunoprecipitation of GRP94 protein from HeLa whole cell extracts using 5 μg of GRP94 antibody [N1N3] (GTX103203). Western blot analysis was performed using GRP94 antibody [N1N3] (GTX103203). EasyBlot anti-Rabbit IgG (GTX221666-01) was used as a secondary reagent.
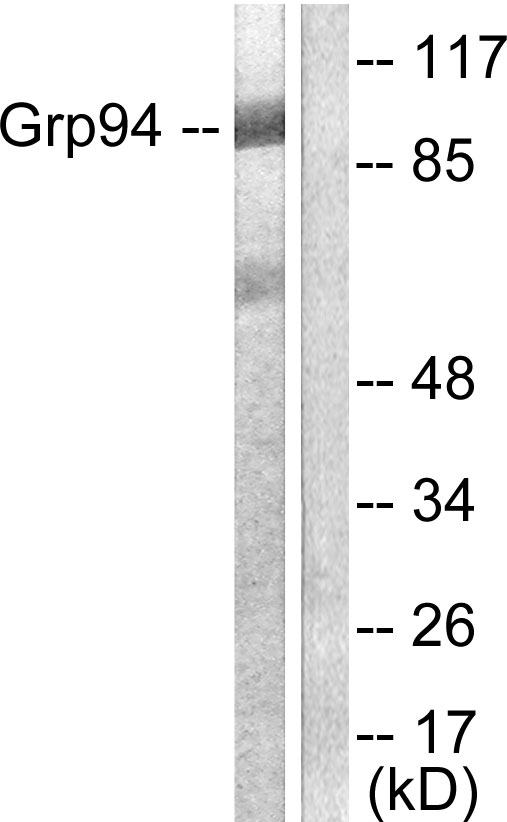
GRP94 antibody [N1N3]
GTX103203
ApplicationsImmunoFluorescence, ImmunoPrecipitation, Western Blot, ImmunoCytoChemistry, ImmunoHistoChemistry, ImmunoHistoChemistry Paraffin
Product group Antibodies
ReactivityHuman, Mouse
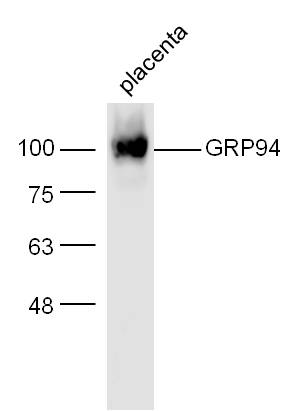
TargetHSP90B1
Overview
- SupplierGeneTex
- Product NameGRP94 antibody [N1N3]
- Delivery Days Customer9
- Application Supplier NoteWB: 1:500-1:10000. ICC/IF: 1:100-1:1000. IHC-P: 1:100-1:1000. IP: 1:100-1:500. *Optimal dilutions/concentrations should be determined by the researcher.Not tested in other applications.
- ApplicationsImmunoFluorescence, ImmunoPrecipitation, Western Blot, ImmunoCytoChemistry, ImmunoHistoChemistry, ImmunoHistoChemistry Paraffin
- CertificationResearch Use Only
- ClonalityPolyclonal
- Concentration0.95 mg/ml
- ConjugateUnconjugated
- Gene ID7184
- Target nameHSP90B1
- Target descriptionheat shock protein 90 beta family member 1
- Target synonymsECGP, GP96, GRP94, HEL-S-125m, HEL35, TRA1, endoplasmin, 94 kDa glucose-regulated protein, endothelial cell (HBMEC) glycoprotein, epididymis luminal protein 35, epididymis secretory sperm binding protein Li 125m, heat shock protein 90 kDa beta member 1, heat shock protein 90kDa beta (Grp94), member 1, heat shock protein 90kDa beta family member 1, heat shock protein family C member 4, stress-inducible tumor rejection antigen gp96, tumor rejection antigen (gp96) 1, tumor rejection antigen 1
- HostRabbit
- IsotypeIgG
- Protein IDP14625
- Protein NameEndoplasmin
- Scientific DescriptionHSP90 proteins are highly conserved molecular chaperones that have key roles in signal transduction, protein folding, protein degradation, and morphologic evolution. HSP90 proteins normally associate with other cochaperones and play important roles in folding newly synthesized proteins or stabilizing and refolding denatured proteins after stress. HSP90B1 is an endoplasmic reticulum HSP90 protein. Other HSP90 proteins are found in cytosol (see HSP90AA1; MIM 140571) and mitochondria (TRAP1; MIM 606219) (Chen et al., 2005 [PubMed 16269234]).[supplied by OMIM]
- ReactivityHuman, Mouse
- Storage Instruction-20°C or -80°C,2°C to 8°C
- UNSPSC41116161

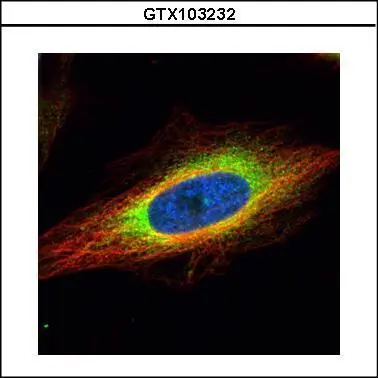
![GRP94 antibody [N1N3] detects GRP94 protein at cytoplasm by immunofluorescent analysis. Sample: HeLa cells were fixed in 4% paraformaldehyde at RT for 15 min. Green: GRP94 protein stained by GRP94 antibody [N1N3] (GTX103203) diluted at 1:500. Blue: Hoechst 33342 staining. GRP94 antibody [N1N3] detects GRP94 protein at cytoplasm by immunofluorescent analysis. Sample: HeLa cells were fixed in 4% paraformaldehyde at RT for 15 min. Green: GRP94 protein stained by GRP94 antibody [N1N3] (GTX103203) diluted at 1:500. Blue: Hoechst 33342 staining.](https://www.genetex.com/upload/website/prouct_img/normal/GTX103203/GTX103203_40016_IFA_w_23060119_461.webp)
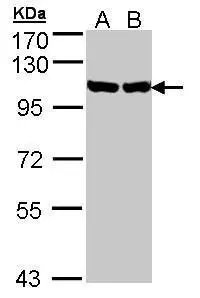
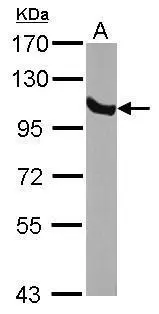
![Wild-type (WT) and GRP94 knockout (KO) 293T cell extracts (30 μg) were separated by 7.5% SDS-PAGE, and the membrane was blotted with GRP94 antibody [N1N3] (GTX103203) diluted at 1:2000. The HRP-conjugated anti-rabbit IgG antibody (GTX213110-01) was used to detect the primary antibody. Wild-type (WT) and GRP94 knockout (KO) 293T cell extracts (30 μg) were separated by 7.5% SDS-PAGE, and the membrane was blotted with GRP94 antibody [N1N3] (GTX103203) diluted at 1:2000. The HRP-conjugated anti-rabbit IgG antibody (GTX213110-01) was used to detect the primary antibody.](https://www.genetex.com/upload/website/prouct_img/normal/GTX103203/GTX103203_40016_20180202_WB_KO_watermark_w_23060119_944.webp)





![ICC/IF analysis of C6 cells using GTX22791 GRP94 antibody [9G10]. Cells were probed without (right) or with(left) an antibody. Green : Primary antibody Blue : Nuclei Red : Actin Fixation : formaldehyde Dilution : 1:100 overnight at 4 oC](https://www.genetex.com/upload/website/prouct_img/normal/GTX22791/GTX22791_452_ICC-IF_w_23060620_104.webp)