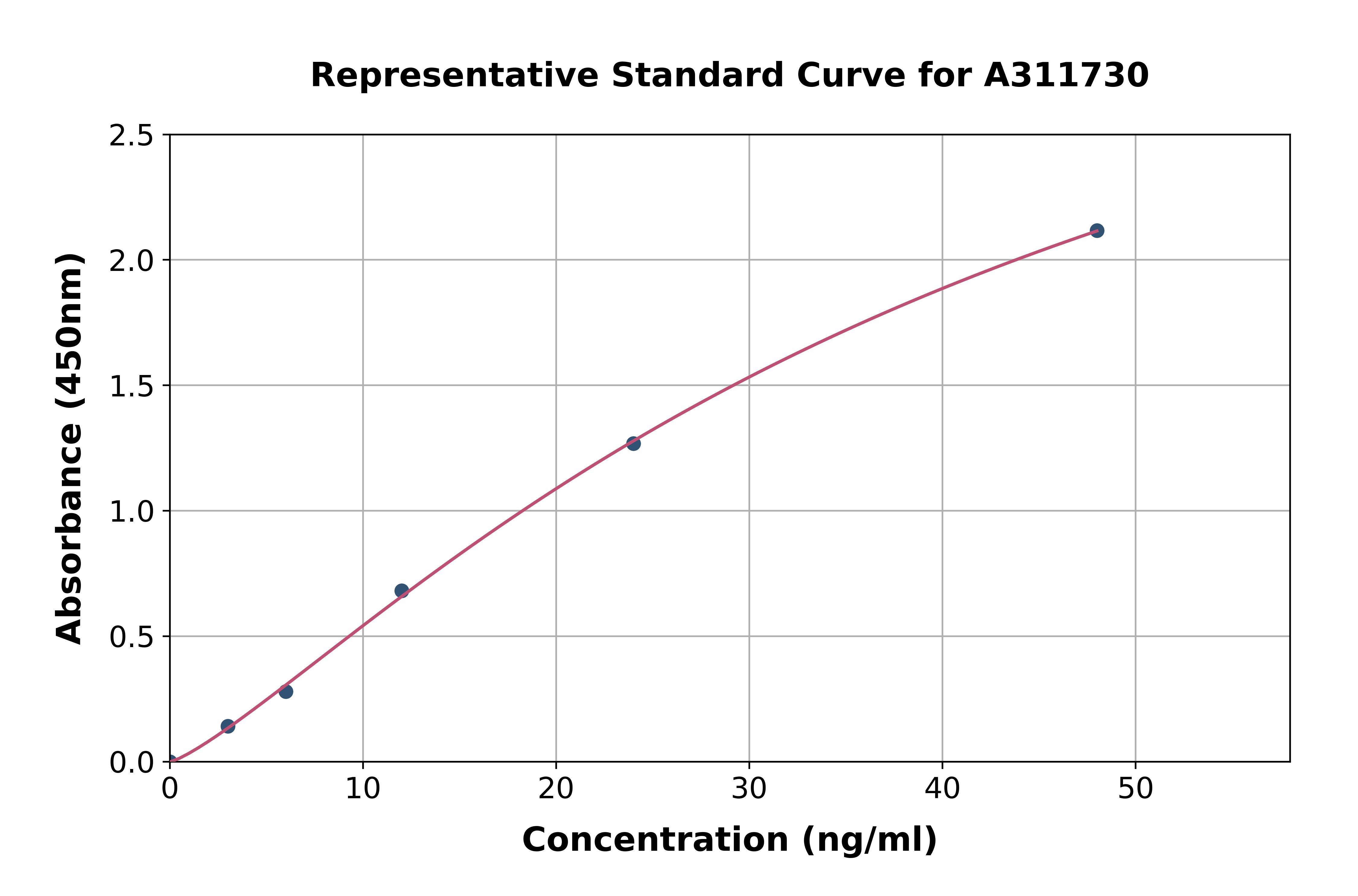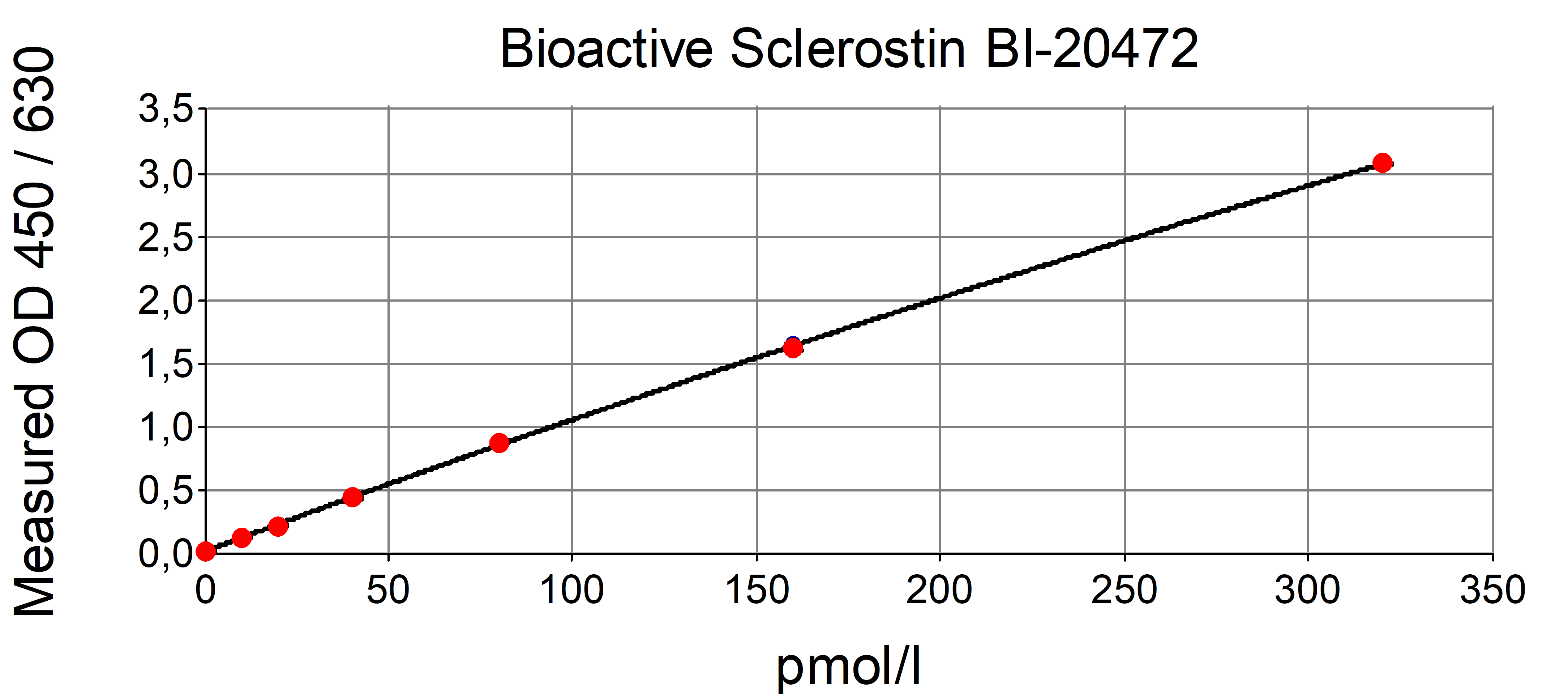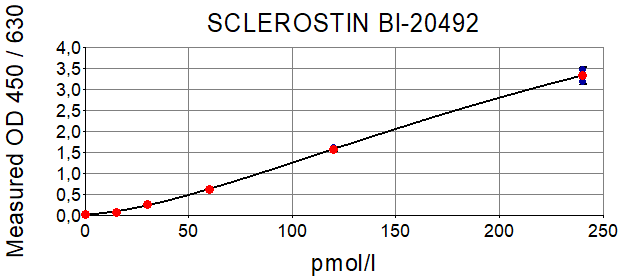
The figure shows a typical standard curve for the human Sclerostin ELISA. The immunoassay is calibrated against human recombinant Sclerostin peptide.
Sclerostin ELISA
BI-20492
Assay Sample Typeserum, EDTA plasma, citrate plasma, heparın plasma, urine, cell culture supernatant
Product group Assays
Overview
- SupplierBiomedica
- Product NameSclerostin ELISA
- Delivery Days Customer7
- ApplicationsELISA
- Applications SupplierELISA
- Assay Detection Range15-240 pmol/l
- Assay Precisionintra-assay: 7%, inter-assay: 10%
- Assay Sample Typeserum, EDTA plasma, citrate plasma, heparın plasma, urine, cell culture supernatant
- Assay Sensitivity3.2 pmol/l
- Assay Test PrincipleSandwich ELISA
- Assay Timeovernight assay
- CertificationResearch Use Only
- Scientific DescriptionProduct Characteristics: The Biomedica human Sclerostin (SOST) Sandwich ELISA kit is a test that is intended for the quantitative measurement of Sclerostin levels in human serum and plasma samples (EDTA, heparin). The Biomedica Sclerostin ELISA is with more than 180 references the most cited Sclerostin ELISA worldwide. Target Information: Canonical Wnt-signalling plays an important role in the regulation of bone homeostasis by promoting the development of osteoblasts. Negative regulators of the Wnt pathway are important therapeutic targets for the treatment of diseases with enhanced bone resorption. One of these molecules is Sclerostin, a 22.5 kD secreted glycoprotein, which acts by binding to the Wnt-coreceptor LRP5 thus preventing the binding of Wnt molecules.
- Storage Instruction2-8°C
- UNSPSC41116133