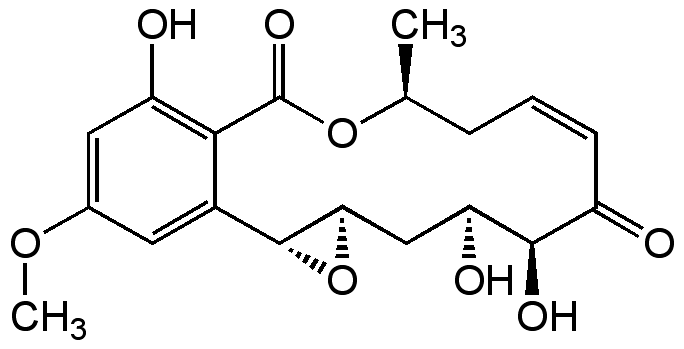
Chemical Structure
Hypothemycin [76958-67-3] [76958-67-3]
BVT-0067
CAS Number76958-67-3
Product group Chemicals
Estimated Purity>98%
Molecular Weight378.4
Overview
- SupplierBioViotica
- Product NameHypothemycin [76958-67-3] [76958-67-3]
- Delivery Days Customer2
- CAS Number76958-67-3
- CertificationResearch Use Only
- Estimated Purity>98%
- Hazard InformationWarning
- Molecular FormulaC19H22O8
- Molecular Weight378.4
- Scientific DescriptionAntifungal. Cytotoxic against some tumor cell lines, partly attributed to inhibition of Ras-inducible genes. Inhibits proliferation of mouse and human T cells. Modulates production of cytokines during T cell activation. Facilitates the ubiquitinylation process of cyclin D1. Potent and selective threonine/tyrosine-specific kinase, MEK and other protein kinases inhibitor in both in vitro and in vivo studies. - Chemical. CAS: 76958-67-3. Formula: C19H22O8. MW: 378.4. Isolated from Phoma sp. Antifungal. Cytotoxic against some tumor cell lines, partly attributed to inhibition of Ras-inducible genes. Inhibits proliferation of mouse and human T cells. Modulates production of cytokines during T cell activation. Facilitates the ubiquitinylation process of cyclin D1. Potent and selective threonine/tyrosine-specific kinase, MEK and other protein kinases inhibitor in both in vitro and in vivo studies.
- SMILESCOC1=CC(O)=C2C(=C1)[C@H]1O[C@@H]1C[C@H](O)[C@H](O)C(=O)\C=C/C[C@H](C)OC2=O
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200

![Hypothemycin [76958-67-3] [76958-67-3]](https://www.targetmol.com/group3/M00/35/8B/CgoaEGayI7GELqHkAAAAABCd8w0234.png)