![ICC/IF analysis of PTK cells using GTX22811 Importin beta 1 antibody [3E9]. ICC/IF analysis of PTK cells using GTX22811 Importin beta 1 antibody [3E9].](https://www.genetex.com/upload/website/prouct_img/normal/GTX22811/GTX22811_1999_ICC-IF_w_23060620_465.webp)
ICC/IF analysis of PTK cells using GTX22811 Importin beta 1 antibody [3E9].
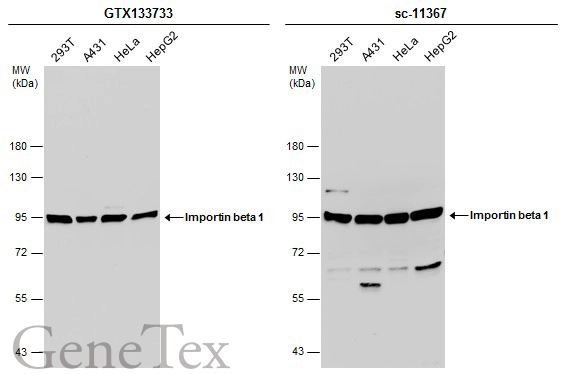
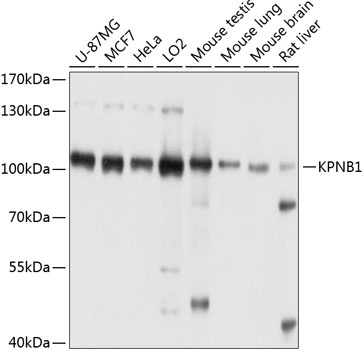
Importin beta 1 antibody [3E9]
GTX22811
ApplicationsFlow Cytometry, ImmunoFluorescence, ImmunoPrecipitation, Western Blot, ChIP Chromatin ImmunoPrecipitation, ImmunoCytoChemistry, ImmunoHistoChemistry, Neutralisation/Blocking
Product group Antibodies
ReactivityBovine, Canine, Human, Mouse, Porcine, Rat
TargetKPNB1
Overview
- SupplierGeneTex
- Product NameImportin beta 1 antibody [3E9]
- Delivery Days Customer9
- Application Supplier NoteWB: 1:5,000. ICC/IF: 1:1000. FACS: 1 microg/test. *Optimal dilutions/concentrations should be determined by the researcher.Not tested in other applications.
- ApplicationsFlow Cytometry, ImmunoFluorescence, ImmunoPrecipitation, Western Blot, ChIP Chromatin ImmunoPrecipitation, ImmunoCytoChemistry, ImmunoHistoChemistry, Neutralisation/Blocking
- CertificationResearch Use Only
- ClonalityMonoclonal
- Concentration1 mg/ml
- ConjugateUnconjugated
- Gene ID3837
- Target nameKPNB1
- Target descriptionkaryopherin subunit beta 1
- Target synonymsIMB1, IPO1, IPOB, Impnb, NTF97, importin subunit beta-1, PTAC97, importin 1, importin 90, importin beta-1 subunit, karyopherin (importin) beta 1, nuclear factor p97, pore targeting complex 97 kDa subunit
- HostMouse
- IsotypeIgG2a
- Protein IDQ14974
- Protein NameImportin subunit beta-1
- Scientific DescriptionNucleocytoplasmic transport, a signal- and energy-dependent process, takes place through nuclear pore complexes embedded in the nuclear envelope. The import of proteins containing a nuclear localization signal (NLS) requires the NLS import receptor, a heterodimer of importin alpha and beta subunits also known as karyopherins. Importin alpha binds the NLS-containing cargo in the cytoplasm and importin beta docks the complex at the cytoplasmic side of the nuclear pore complex. In the presence of nucleoside triphosphates and the small GTP binding protein Ran, the complex moves into the nuclear pore complex and the importin subunits dissociate. Importin alpha enters the nucleoplasm with its passenger protein and importin beta remains at the pore. Interactions between importin beta and the FG repeats of nucleoporins are essential in translocation through the pore complex. The protein encoded by this gene is a member of the importin beta family. Two transcript variants encoding different isoforms have been found for this gene. [provided by RefSeq, Feb 2013]
- ReactivityBovine, Canine, Human, Mouse, Porcine, Rat
- Storage Instruction-20°C or -80°C,2°C to 8°C
- UNSPSC12352203
References
- Tsuchiya K, Hayashi H, Nishina M, et al. Ran-GTP Is Non-essential to Activate NuMA for Mitotic Spindle-Pole Focusing but Dynamically Polarizes HURP Near Chromosomes. Curr Biol. 2021,31(1):115-127.e3. doi: 10.1016/j.cub.2020.09.091Read this paper
- De Munter S, Van der Hoeven G, Bollen M. RepoMan stimulates the chromosome-dependent pathway of microtubule assembly. Cell Cycle. 2020,19(22):3029-3041. doi: 10.1080/15384101.2020.1830607Read this paper
- Jacob JT, Nair RR, Poll BG, et al. Keratin 17 regulates nuclear morphology and chromatin organization. J Cell Sci. 2020,133(20). doi: 10.1242/jcs.254094Read this paper
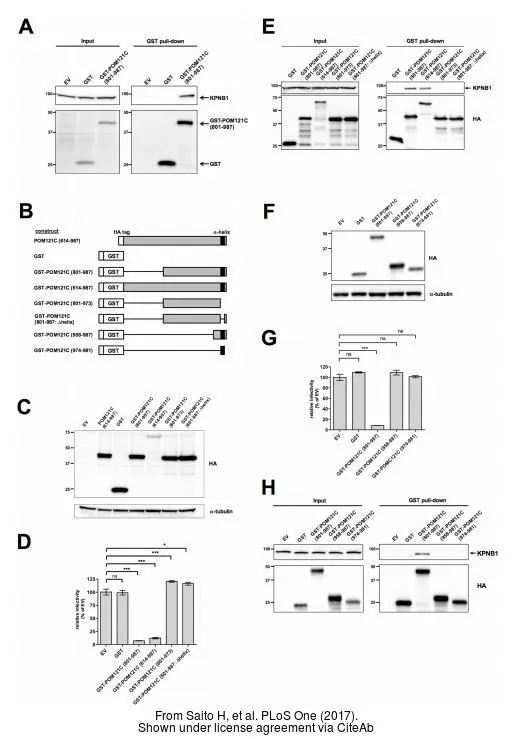
- Saito H, Takeuchi H, Masuda T, et al. N-terminally truncated POM121C inhibits HIV-1 replication. PLoS One. 2017,12(9):e0182434. doi: 10.1371/journal.pone.0182434Read this paper
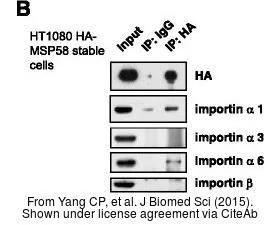
- Yang CP, Chiang CW, Chen CH, et al. Identification and characterization of nuclear and nucleolar localization signals in 58-kDa microspherule protein (MSP58). J Biomed Sci. 2015,22(1):33. doi: 10.1186/s12929-015-0136-0Read this paper

![FACS analysis of NIH-3T3 cells using GTX22811 Importin beta 1 antibody [3E9] compared to an isotype control (blue). Dilution : 1 ug/test for 40 min at room temperature Fixation : 2% paraformaldehyde FACS analysis of NIH-3T3 cells using GTX22811 Importin beta 1 antibody [3E9] compared to an isotype control (blue). Dilution : 1 ug/test for 40 min at room temperature Fixation : 2% paraformaldehyde](https://www.genetex.com/upload/website/prouct_img/normal/GTX22811/GTX22811_155_FACS_w_23060620_848.webp)
![FACS analysis of Jurkat cells using GTX22811 Importin beta 1 antibody [3E9] compared to an isotype control (blue). Dilution : 1 ug/test for 40 min at room temperature Fixation : 2% paraformaldehyde FACS analysis of Jurkat cells using GTX22811 Importin beta 1 antibody [3E9] compared to an isotype control (blue). Dilution : 1 ug/test for 40 min at room temperature Fixation : 2% paraformaldehyde](https://www.genetex.com/upload/website/prouct_img/normal/GTX22811/GTX22811_154_FACS_w_23060620_351.webp)
![FACS analysis of PC12 cells using GTX22811 Importin beta 1 antibody [3E9] compared to an isotype control (blue). Dilution : 1 ug/test for 40 min at room temperature Fixation : 2% paraformaldehyde FACS analysis of PC12 cells using GTX22811 Importin beta 1 antibody [3E9] compared to an isotype control (blue). Dilution : 1 ug/test for 40 min at room temperature Fixation : 2% paraformaldehyde](https://www.genetex.com/upload/website/prouct_img/normal/GTX22811/GTX22811_156_FACS_w_23060620_546.webp)
![ICC/IF analysis of HMVEC Cells using GTX22811 Importin beta 1 antibody [3E9]. ICC/IF analysis of HMVEC Cells using GTX22811 Importin beta 1 antibody [3E9].](https://www.genetex.com/upload/website/prouct_img/normal/GTX22811/GTX22811_494_ICC-IF_w_23060620_113.webp)
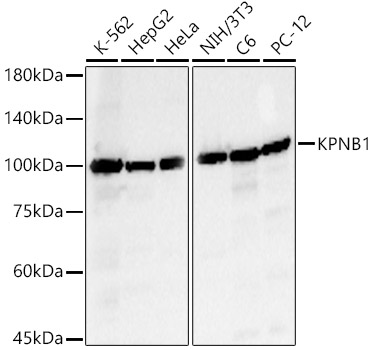
![WB analysis of 25 ug of U251 (lane 1), HepG2 (lane 2) and C6 (lane 3) cell lysates using GTX22811 Importin beta 1 antibody [3E9]. Dilution : 1:50 WB analysis of 25 ug of U251 (lane 1), HepG2 (lane 2) and C6 (lane 3) cell lysates using GTX22811 Importin beta 1 antibody [3E9]. Dilution : 1:50](https://www.genetex.com/upload/website/prouct_img/normal/GTX22811/GTX22811_1605_WB_w_23060620_603.webp)