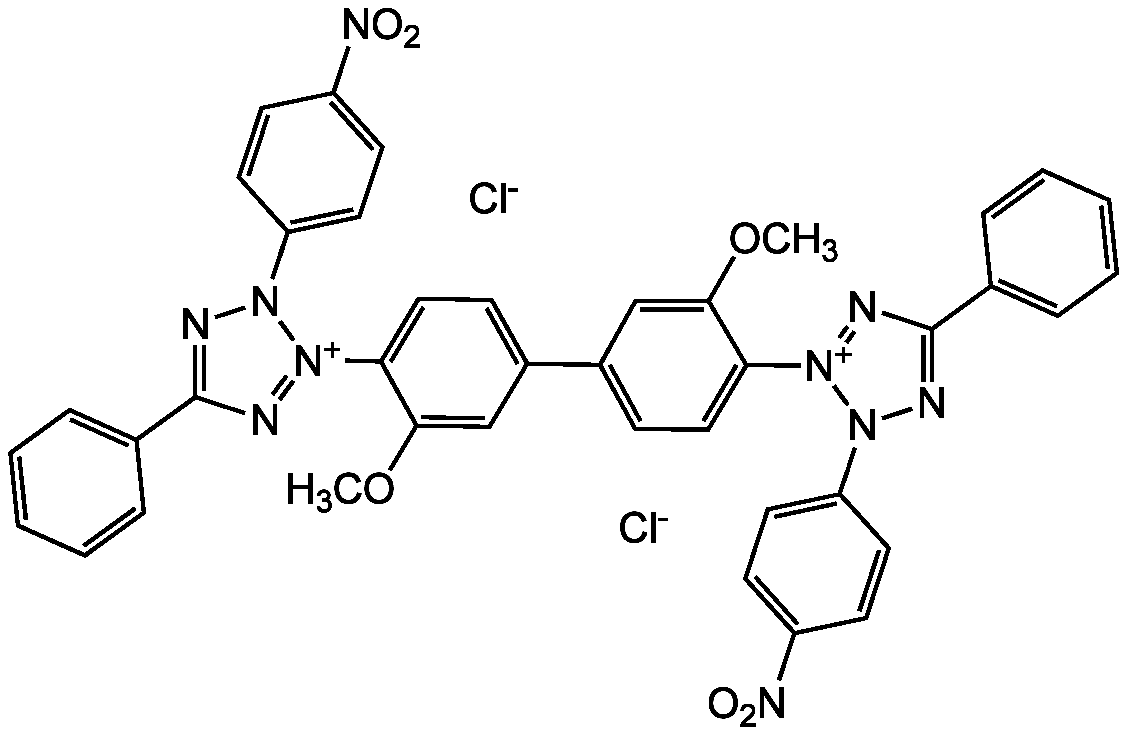
Chemical Structure
Nitrotetrazolium blue chloride [298-83-9]
CDX-N0009
CAS Number298-83-9
Product group Chemicals
Estimated Purity≥95%
Molecular Weight817.2
Overview
- SupplierChemodex
- Product NameNitrotetrazolium blue chloride [298-83-9]
- Delivery Days Customer2
- CAS Number298-83-9
- CertificationResearch Use Only
- Estimated Purity≥95%
- Hazard InformationWarning
- Molecular FormulaC40H30Cl2N10O6
- Molecular Weight817.2
- Scientific DescriptionChemical. CAS: 298-83-9. Formula: C40H30Cl2N10O6. MW: 817.2. Synthetic. NADPH-diaphorase substrate that competitively inhibits NOS (nitric oxide synthase). Well-known scavenger of superoxide anions. Dye that is used for detection of alkaline phosphatase in combination with 5-bromo-4-chloro-3-indoxyl phosphate (BCIP). This substrate system produces an insoluble NBT diformazan end product that is blue in color and can be observed visually. When used with BCIP, it is suitable for detection of alkaline phosphatase in western blots, for immunohistological staining procedures and for colorimetric indication of bacterial infection in blood samples. Used as a redox indicator for enzymatic reactions including dehydrogenases, threonine deaminase, glucose-6-phosphate dehydrogenase, phosphofructokinase on polyacrylamide gels, oxidases on polyacrylamide gels and pentose shunt dehydrogenses. The NBT/BCIP reaction is also used for colorimetric/spectrophotometric activity assays of oxidoreductases. One application is in activity stains in gel electrophoresis, such as with the mitochondrial electron transport chain complexes. - NADPH-diaphorase substrate that competitively inhibits NOS (nitric oxide synthase). Well-known scavenger of superoxide anions. Dye that is used for detection of alkaline phosphatase in combination with 5-bromo-4-chloro-3-indoxyl phosphate (BCIP). This substrate system produces an insoluble NBT diformazan end product that is blue in color and can be observed visually. When used with BCIP, it is suitable for detection of alkaline phosphatase in western blots, for immunohistological staining procedures and for colorimetric indication of bacterial infection in blood samples. Used as a redox indicator for enzymatic reactions including dehydrogenases, threonine deaminase, glucose-6-phosphate dehydrogenase (G6PDH), phosphofructokinase on polyacrylamide gels, oxidases on polyacrylamide gels and pentose shunt dehydrogenses. The NBT/BCIP reaction is also used for colorimetric/spectrophotometric activity assays of oxidoreductases. One application is in activity stains in gel electrophoresis, such as with the mitochondrial electron transport chain complexes.
- SMILES[Cl-].[Cl-].COC1=CC(=CC=C1[N+]1=NC(=NN1C1=CC=C(C=C1)[N+]([O-])=O)C1=CC=CC=C1)C1=CC=C(C(OC)=C1)[N+]1=NC(=NN1C1=CC=C(C=C1)[N+]([O-])=O)C1=CC=CC=C1
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12162000