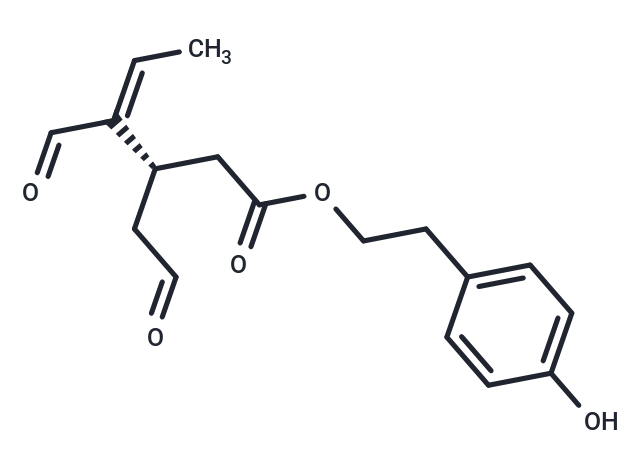
Chemical Structure
Oleocanthal [289030-99-5] [289030-99-5]
AG-CN2-0537
CAS Number289030-99-5
Product group Chemicals
Estimated Purity>97%
Molecular Weight304.3
Overview
- SupplierAdipoGen Life Sciences
- Product NameOleocanthal [289030-99-5] [289030-99-5]
- Delivery Days Customer10
- CAS Number289030-99-5
- CertificationResearch Use Only
- Estimated Purity>97%
- Molecular FormulaC17H20O5
- Molecular Weight304.3
- Scientific DescriptionChemical. CAS: 289030-99-5. Formula: C17H20O5. MW: 304.3. Oleocanthal is a naturally occurring amphiphilic secoiridoid from olive oil, with potent anti-inflammatory, antioxidant, anticancer and neuroprotective properties. Similar to classical non-steroidal anti-inflammatory drugs, it is a non-selective inhibitor of cyclooxygenase (COX-1, COX-2) enzymes in the prostaglandin biosynthesis. It also has been shown to inhibit the NLRP3 inflammasome. Inhibits proliferation, migration and invasion of various cancer cells while leaving healthy cells unharmed. It shows selective cytotoxicity, inducing lysosomal membrane permeabilization in cancer cells. It inhibits the enzymatic activity of mammalian target of rapamycin (mTOR), and the tyrosine kinase receptor c-Met (responsible for proliferation of many cell types) and Hsp90, consequently leading to cell cycle arrest during G1 phase. The neuroprotective activity includes the reduction of beta-amyloid protein accumulation via up-regulation of P-glycoprotein and LRP1, altering the fibrillization of tau proteins, key factors at the basis of neurodegenerative diseases and reducing oxidative stress. Activator of the TRPA1 ion channel, responsible for the burning sensation that occurs in the back of the throat when consuming such oil. Shows antimicrobial activities. - Oleocanthal is a naturally occurring amphiphilic secoiridoid from olive oil, with potent anti-inflammatory, antioxidant, anticancer and neuroprotective properties. Similar to classical non-steroidal anti-inflammatory drugs, it is a non-selective inhibitor of cyclooxygenase (COX-1, COX-2) enzymes in the prostaglandin biosynthesis. It also has been shown to inhibit the NLRP3 inflammasome. Inhibits proliferation, migration and invasion of various cancer cells while leaving healthy cells unharmed. It shows selective cytotoxicity, inducing lysosomal membrane permeabilization in cancer cells. It inhibits the enzymatic activity of mammalian target of rapamycin (mTOR), and the tyrosine kinase receptor c-Met (responsible for proliferation of many cell types) and Hsp90, consequently leading to cell cycle arrest during G1 phase. The neuroprotective activity includes the reduction of beta-amyloid protein accumulation via up-regulation of P-glycoprotein and LRP1, altering the fibrillization of tau proteins, key factors at the basis of neurodegenerative diseases and reducing oxidative stress. Activator of the TRPA1 ion channel, responsible for the burning sensation that occurs in the back of the throat when consuming such oil. Shows antimicrobial activities.
- SMILESOC1=CC=C(CCOC(C[C@H](CC([H])=O)/C(C([H])=O)=C\C)=O)C=C1
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200