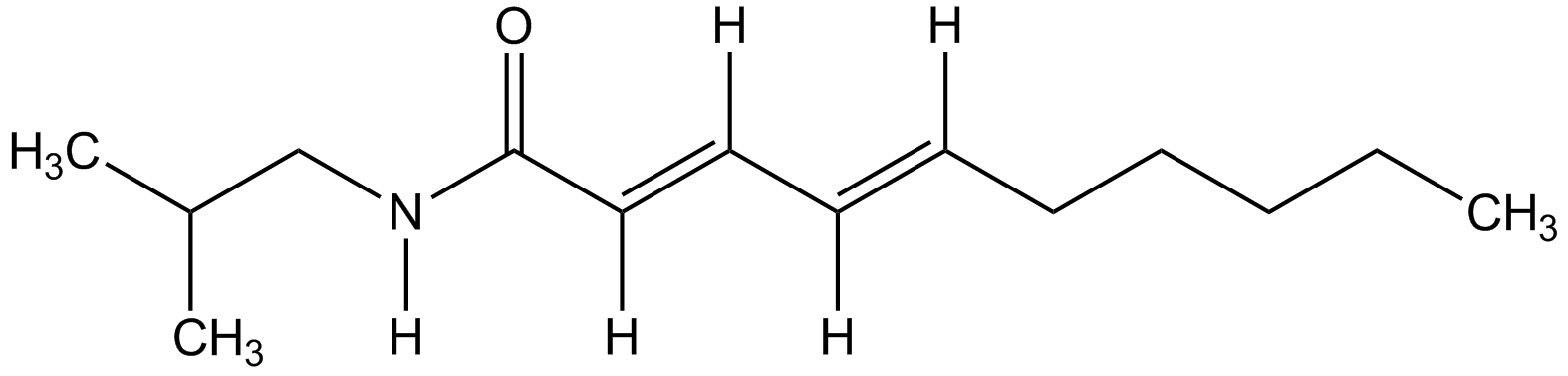
Chemical Structure
Pellitorine [18836-52-7] [18836-52-7]
AG-CN2-0009
CAS Number18836-52-7
Product group Chemicals
Estimated Purity>97%
Molecular Weight223.4
Overview
- SupplierAdipoGen Life Sciences
- Product NamePellitorine [18836-52-7] [18836-52-7]
- Delivery Days Customer10
- CAS Number18836-52-7
- CertificationResearch Use Only
- Estimated Purity>97%
- Molecular FormulaC14H25NO
- Molecular Weight223.4
- Scientific DescriptionChemical. CAS: 18836-52-7. Formula: C14H25NO. MW: 223.4. Synthetic. Originally isolated from roots of Anacyclus pryrethrum and fruits of Piper nigrum. Tingling-inducing agent. Excellent stable model compound for sensory studies. Exerts same profile as the unstable compound hydroxy-alpha-sanshool. Shows larvicidal, antimycobacterial and antituberculosis activity. alpha-Glucosidase inhibitor used in diabetes mellitus, cancer, infection and inflammatory research. ACAT (Acyl-CoA cholesteryl acyl transferase) inhibitor. Potential anti-cancer lead compound. Anti-thrombotic. Anti-septic. Antiprotozoal, antimalarial activity. - Tingling-inducing agent. Excellent stable model compound for sensory studies [3]. Exerts same profile as the unstable compound hydroxy-alpha-sanshool. Shows larvicidal, antimycobacterial and antituberculosis activity [1, 2, 4]. alpha-Glucosidase inhibitor used in diabetes mellitus, cancer, infection and inflammatory research [5]. ACAT (Acyl-CoA cholesteryl acyl transferase) inhibitor [6]. Potential anti-cancer lead compound [7]. Anti-thrombotic [8]. Anti-septic [9]. Antiprotozoal, antimalarial activity [10]. Antagonist of the TRPV1 [11].
- SMILES[H]N(CC(C)C)C(=O)C(\[H])=C(/[H])\C(\[H])=C(/[H])CCCCC
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200

![Pellitorine [18836-52-7] [18836-52-7]](https://www.targetmol.com/group3/M00/36/04/CgoaEGayND6EJ5lTAAAAAIveiK8233.png)