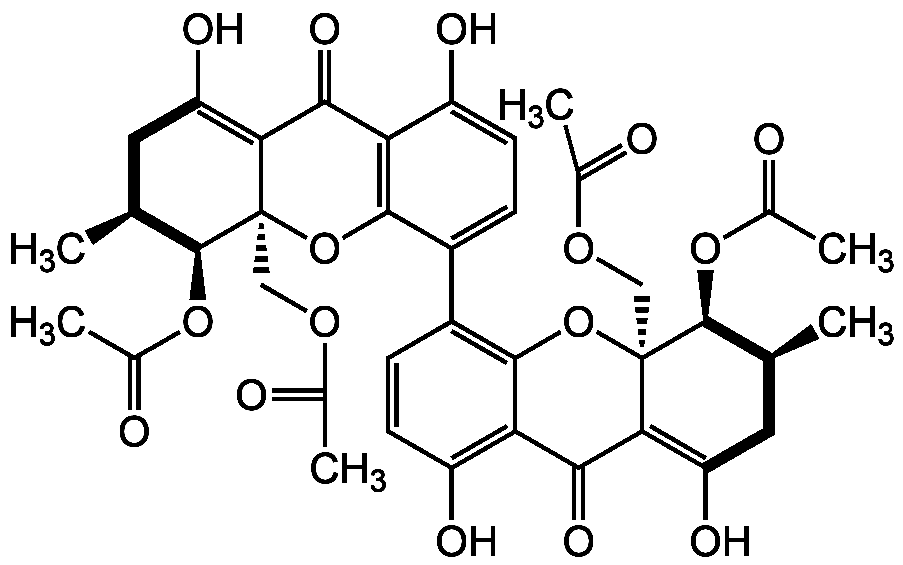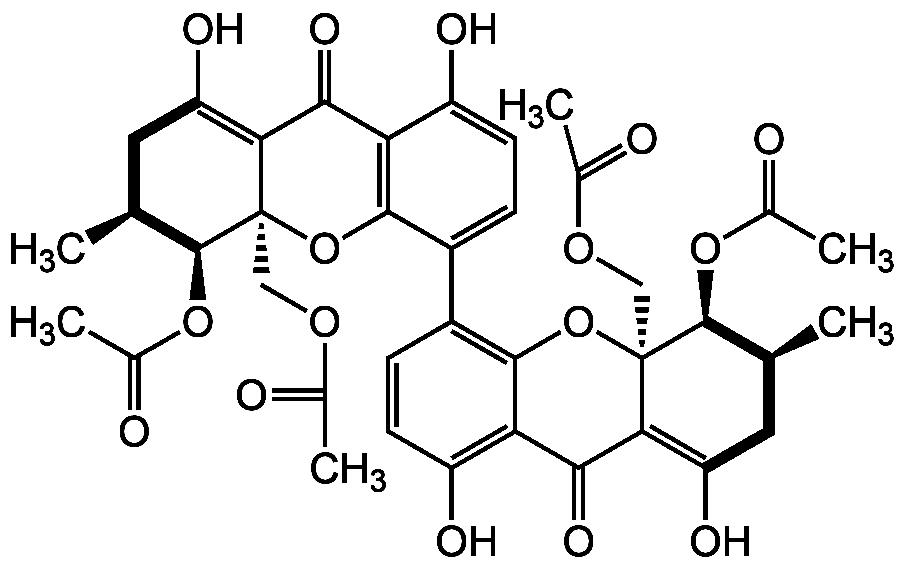
Chemical Structure
Phomoxanthone A
AG-CN2-0017
CAS Number359844-69-2
Product group Chemicals
Estimated Purity>97%
Molecular Weight750.7
Overview
- SupplierAdipoGen Life Sciences
- Product NamePhomoxanthone A
- Delivery Days Customer10
- CAS Number359844-69-2
- CertificationResearch Use Only
- Estimated Purity>97%
- Molecular FormulaC38H38O16
- Molecular Weight750.7
- Scientific DescriptionAnticancer compound. Shows antimalarial and antitubercular activity. Cytotoxic against several cancer cell lines. Antibacterial against Gram-positive bacteria and antifungal. Activator of murine T lymphocytes, NK cells and macrophages. Mitochondrial toxin with a mode of action distinct from known electron transport chain (ETC) inhibitors, OXPHOS uncouplers and ionophores. Shows rapid inhibition of both ETC and DeltaPsim, the release of mitochondrial Ca2+ and fission of the inner but not the outer mitochondrial membrane independent from the mitochondrial fission and fusion regulators DRP1 and OPA1. - Chemical. CAS: 359844-69-2. Formula: C38H38O16. MW: 750.7. Isolated from fungus Phoma sp. Anticancer compound. Shows antimalarial and antitubercular activity. Cytotoxic against several cancer cell lines. Antibacterial against Gram-positive bacteria and antifungal. Activator of murine T lymphocytes, NK cells and macrophages.
- SMILESC[C@@H]1CC(O)=C2C(=O)C3=C(O[C@]2(COC(C)=O)[C@@H]1OC(C)=O)C(=CC=C3O)C1=C2O[C@]3(COC(C)=O)[C@H](OC(C)=O)[C@H](C)CC(O)=C3C(=O)C2=C(O)C=C1
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200