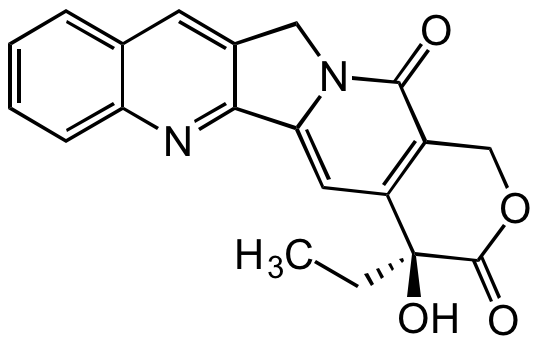
Chemical Structure
(S)-(+)-Camptothecin [7689-03-4]
AG-CN2-0463
CAS Number7689-03-4
Product group Chemicals
Estimated Purity>98%
Molecular Weight348.4
Overview
- SupplierAdipoGen Life Sciences
- Product Name(S)-(+)-Camptothecin [7689-03-4]
- Delivery Days Customer10
- CAS Number7689-03-4
- CertificationResearch Use Only
- Estimated Purity>98%
- Hazard InformationDanger,Excepted quantity
- Molecular FormulaC20H16N2O4
- Molecular Weight348.4
- Scientific DescriptionChemical. CAS: 7689-03-4. Formula: C20H16N2O4. MW: 348.4. Isolated from Camptotheca acuminata. Potent anticancer compound. Cell permeable potent DNA topoisomerase I (Topo I) complex inhibitor. Potent apoptosis inducer. Binds reversibly to the DNA topoisomerase I complex, inhibiting the reassociation of DNA after cleavage by topoisomerase I and traps the enzyme in a covalent linkage with DNA. The enzyme complex is ubiquinated and destroyed by the 26S proteasome, consequently depleting cellular topoisomerase I. Prevents DNA re-ligation and therefore causes DNA damage which results in apoptosis. Inhibits mitochondrial topoisomerase I (mtTop1). Blocks the cell cycle at low dose and induces apoptosis in a large number of normal and tumor cell lines by cell cycle-dependent and cell cycle-independent processes. Antiprotozoal and antimalarial compound. Inhibitor of HIV replication and of other viruses. Suppresses nitric oxide (NO) biosynthesis. Shown to suppress TNF-alpha-induced expression of the inflammasome and cyclooxygenase 2 (COX-2). - Potent anticancer compound. Cell permeable potent DNA topoisomerase I (Topo I) complex inhibitor. Potent apoptosis inducer. Binds reversibly to the DNA topoisomerase I complex, inhibiting the reassociation of DNA after cleavage by topoisomerase I and traps the enzyme in a covalent linkage with DNA. The enzyme complex is ubiquinated and destroyed by the 26S proteasome, consequently depleting cellular topoisomerase I. Prevents DNA re-ligation and therefore causes DNA damage which results in apoptosis. Inhibits mitochondrial topoisomerase I (mtTop1). Blocks the cell cycle at low dose and induces apoptosis in a large number of normal and tumor cell lines by cell cycle-dependent and cell cycle-independent processes. Antiprotozoal and antimalarial compound. Inhibitor of HIV replication and of other viruses. Suppresses nitric oxide (NO) biosynthesis. Shown to suppress TNF-alpha-induced expression of the inflammasome and cyclooxygenase 2 (COX-2).
- SMILESCC[C@@]1(O)C(=O)OCC2=C1C=C1N(CC3=CC4=CC=CC=C4N=C13)C2=O
- Storage Instruction2°C to 8°C,RT
- UN NumberUN 2811
- UNSPSC12352200


![Camptothecin [7689-03-4]](https://www.targetmol.com/group3/M00/03/12/CgoaEWY7QNyEIBJWAAAAAMrb-_s319.png)
