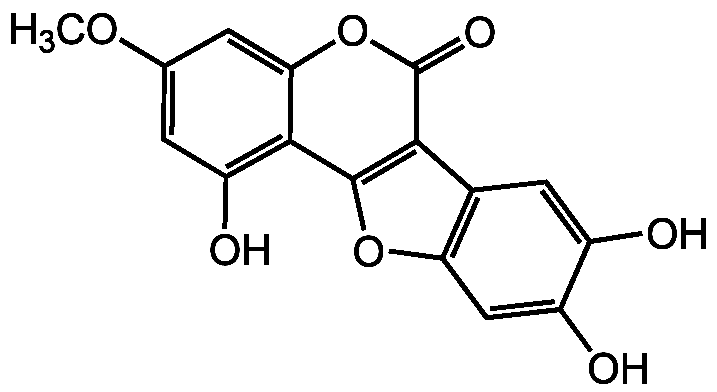
Chemical Structure
Wedelolactone [524-12-9] [524-12-9]
AG-CN2-0424
CAS Number524-12-9
Product group Chemicals
Estimated Purity>98%
Molecular Weight314.2
Overview
- SupplierAdipoGen Life Sciences
- Product NameWedelolactone [524-12-9] [524-12-9]
- Delivery Days Customer10
- CAS Number524-12-9
- CertificationResearch Use Only
- Estimated Purity>98%
- Hazard InformationWarning
- Molecular FormulaC16H10O7
- Molecular Weight314.2
- Scientific DescriptionCell permeable, selective and irreversible IKKalpha and beta kinase activity inhibitor [2]. NF-kappaB inhibitor. Blocks phosphorylation and degradation of IkappaBalpha [2]. Antihepatotoxic. 5-Lipoxygenase (5-LOX) inhibitor [1, 2]. Anti-inflammatory [2]. HIV-1 integrase inhibitor [3]. Anticancer and antiproliferative compound [4, 5]. Modulates androgen receptor (AR) activation [4]. Apoptosis inducer via downregulation of PKCepsilon [5, 9]. DNA topoisomerase IIalpha inhibitor [6]. G protein-coupled receptor-35 (GPR35) agonist [7]. Inhibits adipogenic differentiation of mesenchymal stem cells (hAMSCs) through ERK pathway [8]. STAT1 protein dephosphorylation inhibitor [10]. Antifibrotic [11]. - Chemical. CAS: 524-12-9. Formula: C16H10O7. MW: 314.2. Isolated from Eclipta prostrata. Cell permeable, selective and irreversible IKKalpha and beta kinase activity inhibitor. NF-kappaB inhibitor. Blocks phosphorylation and degradation of IkappaBalpha. Antihepatotoxic. 5-Lipoxygenase (5-LOX) inhibitor. Anti-inflammatory. HIV-1 integrase inhibitor. Anticancer and antiproliferative compound. Modulates androgen receptor (AR) activation. Apoptosis inducer via downregulation of PKCepsilon. DNA topoisomerase IIalpha inhibitor. G protein-coupled receptor-35 (GPR35) agonist. Inhibits adipogenic differentiation of mesenchymal stem cells (hAMSCs) through ERK pathway. STAT1 protein dephosphorylation inhibitor. Antifibrotic.
- SMILESCOC1=CC(O)=C2C3=C(C4=C(O3)C=C(O)C(O)=C4)C(=O)OC2=C1
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200

![Wedelolactone [524-12-9] [524-12-9]](https://www.targetmol.com/group3/M00/37/CC/CgoaEWayUhuECiq6AAAAALVGltk911.png)