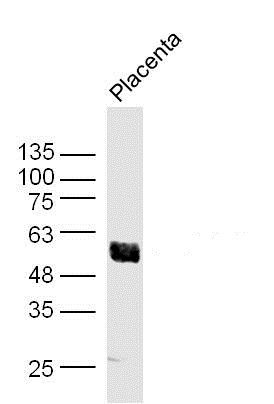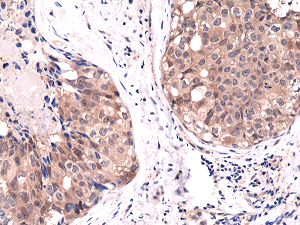
Immunohistochemical staining of formalin fixed and paraffin embedded human breast cancer tissue sections using Anti-Akt1 RM252 at a 1:1000 dilution.
anti-Akt1 (human), Rabbit Monoclonal (RM252)
REV-31-1132-00
ApplicationsWestern Blot, ImmunoHistoChemistry
Product group Antibodies
ReactivityHuman
TargetAKT1
Overview
- SupplierRevMAb Biosciences
- Product Nameanti-Akt1 (human), Rabbit Monoclonal (RM252)
- Delivery Days Customer2
- ApplicationsWestern Blot, ImmunoHistoChemistry
- CertificationResearch Use Only
- ClonalityMonoclonal
- Clone IDRM252
- Gene ID207
- Target nameAKT1
- Target descriptionAKT serine/threonine kinase 1
- Target synonymsAKT, PKB, PKB-ALPHA, PRKBA, RAC, RAC-ALPHA, RAC-alpha serine/threonine-protein kinase, AKT1m, PKB alpha, RAC-PK-alpha, protein kinase B alpha, proto-oncogene c-Akt, rac protein kinase alpha, serine-threonine protein kinase, v-akt murine thymoma viral oncogene homolog 1, v-akt murine thymoma viral oncogene-like protein 1
- HostRabbit
- IsotypeIgG
- Protein IDP31749
- Protein NameRAC-alpha serine/threonine-protein kinase
- Scientific DescriptionAkt, also referred to as PKB or Rac, plays a critical role in controlling survival and apoptosis. This protein kinase is activated at 2 phosphorylation sites Thr308 and Ser473. Akt promotes cell survival by inhibiting apoptosis through phosphorylation and inactivation of several targets, including Bad, forkhead transcription factors, c-Raf and caspase-9. In addition to its role in survival and glycogen synthesis, Akt is involved in cell cycle regulation. Akt also plays a critical role in cell growth by directly phosphorylating mTOR in a rapamycin-sensitive complex containing raptor. Mutation of the glutamic acid at residue 17 to lysine (E17K) of Akt was initially identified in human breast, colorectal and ovarian cancers. This conserved glutamic acid residue is located at the lipid-binding pocket of the Akt plextrin homology domain. The E17K mutation increases the affinity between Akt and phospholipids at the plasma membrane, leading to increased Akt recruitment, super-activation of the Akt pathway, cellular transformation and tumor formation. Additional studies detect the presence of the Akt (E17K) mutation in multiple cancers, including lung cancer, prostate cancer, endometrial carcinoma and several melanomas. - Recombinant Antibody. This antibody reacts to human Akt1. This antibody may also react to bovine, mouse or rat Akt1, as predicted by immunogen homology. Applications: WB, IHC. Source: Rabbit. Liquid. 50% Glycerol/PBS with 1% BSA and 0.09% sodium azide. Akt, also referred to as PKB or Rac, plays a critical role in controlling survival and apoptosis. This protein kinase is activated at 2 phosphorylation sites Thr308 and Ser473. Akt promotes cell survival by inhibiting apoptosis through phosphorylation and inactivation of several targets, including Bad, forkhead transcription factors, c-Raf and caspase-9. In addition to its role in survival and glycogen synthesis, Akt is involved in cell cycle regulation. Akt also plays a critical role in cell growth by directly phosphorylating mTOR in a rapamycin-sensitive complex containing raptor. Mutation of the glutamic acid at residue 17 to lysine (E17K) of Akt was initially identified in human breast, colorectal and ovarian cancers. This conserved glutamic acid residue is located at the lipid-binding pocket of the Akt plextrin homology domain. The E17K mutation increases the affinity between Akt and phospholipids at the plasma membrane, leading to increased Akt recruitment, super-activation of the Akt pathway, cellular transformation and tumor formation. Additional studies detect the presence of the Akt (E17K) mutation in multiple cancers, including lung cancer, prostate cancer, endometrial carcinoma and several melanomas.
- ReactivityHuman
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC41116161