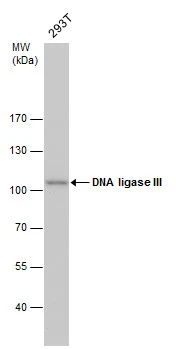
DNA ligase III antibody detects DNA ligase III protein by western blot analysis. Whole cell extracts (30 μg) was separated by 7.5% SDS-PAGE, and the membrane was blotted with DNA ligase III antibody (GTX70143) diluted by 1:500. The HRP-conjugated anti-mouse IgG antibody (GTX213111-01) was used to detect the primary antibody.
DNA ligase III antibody [1F3]
GTX70143
ApplicationsImmunoFluorescence, ImmunoPrecipitation, Western Blot, ImmunoCytoChemistry, Neutralisation/Blocking, Other Application
Product group Antibodies
ReactivityChicken, Human, Mouse
TargetLIG3
Overview
- SupplierGeneTex
- Product NameDNA ligase III antibody [1F3]
- Delivery Days Customer9
- Application Supplier NoteWB: 1:500-1:3000. *Optimal dilutions/concentrations should be determined by the researcher.Not tested in other applications.
- ApplicationsImmunoFluorescence, ImmunoPrecipitation, Western Blot, ImmunoCytoChemistry, Neutralisation/Blocking, Other Application
- CertificationResearch Use Only
- ClonalityMonoclonal
- Clone ID1F3
- Concentration1 mg/ml
- ConjugateUnconjugated
- Gene ID3980
- Target nameLIG3
- Target descriptionDNA ligase 3
- Target synonymsLIG2, LIG3alpha, MTDPS20, DNA ligase 3, ligase II, DNA, ATP-dependent, ligase III, DNA, ATP-dependent, polydeoxyribonucleotide synthase [ATP] 3
- HostMouse
- IsotypeIgG1
- Protein IDP49916
- Protein NameDNA ligase 3
- Scientific DescriptionThis gene is a member of the DNA ligase family. Each member of this family encodes a protein that catalyzes the joining of DNA ends but they each have a distinct role in DNA metabolism. The protein encoded by this gene is involved in excision repair and is located in both the mitochondria and nucleus, with translation initiation from the upstream start codon allowing for transport to the mitochondria and translation initiation from a downstream start codon allowing for transport to the nucleus. Additionally, alternate transcriptional splice variants, encoding different isoforms, have been characterized. [provided by RefSeq, Jul 2008]
- ReactivityChicken, Human, Mouse
- Storage Instruction-20°C or -80°C,2°C to 8°C
- UNSPSC41116161

![DNA ligase III detects DNA ligase III antibody [1F3] protein by western blot analysis. A. 30 μg 293T whole cell lysate/extract B. 30 μg A431 whole cell lysate/extract C. 30 μg HeLa whole cell lysate/extract D. 30 μg HepG2 whole cell lysate/extract 7.5% SDS-PAGE DNA ligase III (GTX70143) dilution: 1:500 The HRP-conjugated anti-mouse IgG antibody (GTX213111-01) was used to detect the primary antibody. DNA ligase III detects DNA ligase III antibody [1F3] protein by western blot analysis. A. 30 μg 293T whole cell lysate/extract B. 30 μg A431 whole cell lysate/extract C. 30 μg HeLa whole cell lysate/extract D. 30 μg HepG2 whole cell lysate/extract 7.5% SDS-PAGE DNA ligase III (GTX70143) dilution: 1:500 The HRP-conjugated anti-mouse IgG antibody (GTX213111-01) was used to detect the primary antibody.](https://www.genetex.com/upload/website/prouct_img/normal/GTX70143/GTX70143_26881_WB_w_23061221_193.webp)



![HepG2 whole cell and nuclear extracts (30 μg) were separated by 7.5% SDS-PAGE, and the membrane was blotted with DNA ligase III antibody [HL2280] (GTX638333) diluted at 1:1000. The HRP-conjugated anti-rabbit IgG antibody (GTX213110-01) was used to detect the primary antibody.](https://www.genetex.com/upload/website/prouct_img/normal/GTX638333/GTX638333_T-44977_20230317_WB_Fraction_23032022_926.webp)
![HepG2 whole cell and nuclear extracts (30 μg) were separated by 5% SDS-PAGE, and the membrane was blotted with DNA ligase III antibody [C2C3], C-term (GTX103197) diluted at 1:500. The HRP-conjugated anti-rabbit IgG antibody (GTX213110-01) was used to detect the primary antibody.](https://www.genetex.com/upload/website/prouct_img/normal/GTX103197/GTX103197_44349_20210702_WB_Fraction_w_23060119_184.webp)