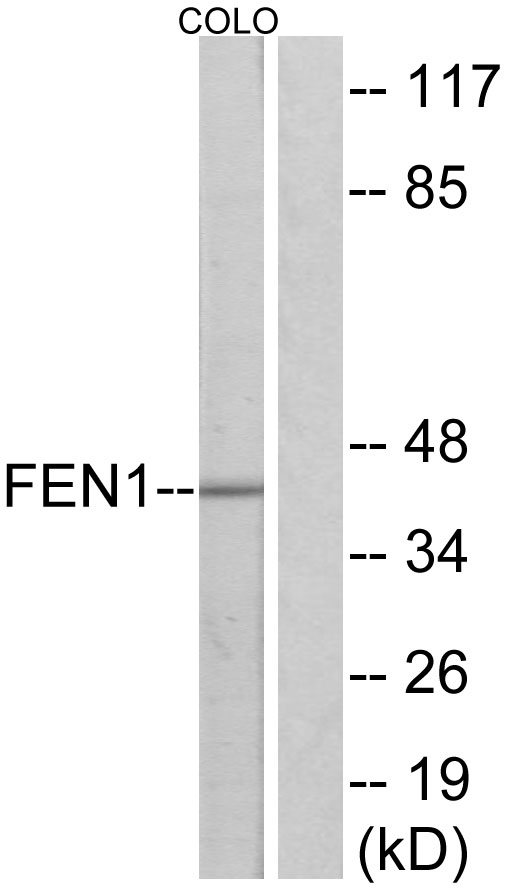
FEN1 antibody [4E7]
GTX70185
ApplicationsImmunoFluorescence, ImmunoPrecipitation, Western Blot, ChIP Chromatin ImmunoPrecipitation, ImmunoCytoChemistry
Product group Antibodies
TargetFEN1
Overview
- SupplierGeneTex
- Product NameFEN1 antibody [4E7]
- Delivery Days Customer9
- Application Supplier NoteWB: 1:500-1:3000. *Optimal dilutions/concentrations should be determined by the researcher.Not tested in other applications.
- ApplicationsImmunoFluorescence, ImmunoPrecipitation, Western Blot, ChIP Chromatin ImmunoPrecipitation, ImmunoCytoChemistry
- CertificationResearch Use Only
- ClonalityMonoclonal
- Concentration1.73 mg/ml
- ConjugateUnconjugated
- Gene ID2237
- Target nameFEN1
- Target descriptionflap structure-specific endonuclease 1
- Target synonymsFEN-1, MF1, RAD2, flap endonuclease 1, DNase IV, maturation factor-1
- HostMouse
- IsotypeIgG1
- Protein IDP39748
- Protein NameFlap endonuclease 1
- Scientific DescriptionThe protein encoded by this gene removes 5 overhanging flaps in DNA repair and processes the 5 ends of Okazaki fragments in lagging strand DNA synthesis. Direct physical interaction between this protein and AP endonuclease 1 during long-patch base excision repair provides coordinated loading of the proteins onto the substrate, thus passing the substrate from one enzyme to another. The protein is a member of the XPG/RAD2 endonuclease family and is one of ten proteins essential for cell-free DNA replication. DNA secondary structure can inhibit flap processing at certain trinucleotide repeats in a length-dependent manner by concealing the 5 end of the flap that is necessary for both binding and cleavage by the protein encoded by this gene. Therefore, secondary structure can deter the protective function of this protein, leading to site-specific trinucleotide expansions. [provided by RefSeq, Jul 2008]
- Storage Instruction-20°C or -80°C,2°C to 8°C
- UNSPSC12352203
References
- Hao Q, Zhan C, Lian C, et al. DNA repair mechanisms that promote insertion-deletion events during immunoglobulin gene diversification. Sci Immunol. 2023,8(81):eade1167. doi: 10.1126/sciimmunol.ade1167Read this paper
- Ma Z, Wang W, Wang S, et al. Symmetrical dimethylation of H4R3: A bridge linking DNA damage and repair upon oxidative stress. Redox Biol. 2020,37:101653. doi: 10.1016/j.redox.2020.101653Read this paper
- Li JL, Wang JP, Chang H, et al. FEN1 inhibitor increases sensitivity of radiotherapy in cervical cancer cells. Cancer Med. 2019,8(18):7774-7780. doi: 10.1002/cam4.2615Read this paper
- Kanagaraj A, Sakamoto N, Que L, et al. Different antiviral activities of natural APOBEC3C, APOBEC3G, and APOBEC3H variants against hepatitis B virus. Biochem Biophys Res Commun. 2019,518(1):26-31. doi: 10.1016/j.bbrc.2019.08.003Read this paper
- Kitamura K, Que L, Shimadu M, et al. Flap endonuclease 1 is involved in cccDNA formation in the hepatitis B virus. PLoS Pathog. 2018,14(6):e1007124. doi: 10.1371/journal.ppat.1007124Read this paper
- Li Z, Liu B, Jin W, et al. hDNA2 nuclease/helicase promotes centromeric DNA replication and genome stability. EMBO J. 2018,37(14). doi: 10.15252/embj.201796729Read this paper
- Zhang K, Keymeulen S, Nelson R, et al. Overexpression of Flap Endonuclease 1 Correlates with Enhanced Proliferation and Poor Prognosis of Non-Small-Cell Lung Cancer. Am J Pathol. 2018,188(1):242-251. doi: 10.1016/j.ajpath.2017.09.011Read this paper
- Briest F, Grass I, Sedding D, et al. Mechanisms of Targeting the MDM2-p53-FOXM1 Axis in Well-Differentiated Intestinal Neuroendocrine Tumors. Neuroendocrinology. 2018,107(1):1-23. doi: 10.1159/000481506Read this paper
- Tsai YC, Wang YH, Liu YC. Overexpression of PCNA Attenuates Oxidative Stress-Caused Delay of Gap-Filling during Repair of UV-Induced DNA Damage. J Nucleic Acids. 2017,2017:8154646. doi: 10.1155/2017/8154646Read this paper
- Sami F, Lu X, Parvathaneni S, et al. RECQ1 interacts with FEN-1 and promotes binding of FEN-1 to telomeric chromatin. Biochem J. 2015,468(2):227-44. doi: 10.1042/BJ20141021Read this paper




![ICC/IF analysis of 4% PFA-fixed HeLa cells using GTX66841 FEN1 antibody [7H8]. Dilution : 1:400](https://www.genetex.com/upload/website/prouct_img/normal/GTX66841/GTX66841_20190305_ICCIF_w_23061221_171.webp)