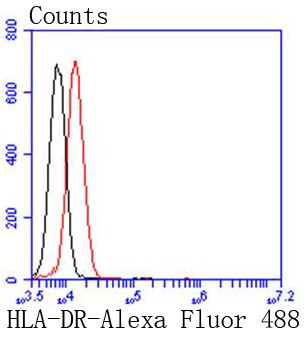
![FACS analysis of human peripheral blood monocytes using GTX01489-10 HLA-DR antibody [LN3] (PE-Cy7). Solid lone : primary antibody Dashed line : isotype control antibody amount : 0.125 microg (5 microl) FACS analysis of human peripheral blood monocytes using GTX01489-10 HLA-DR antibody [LN3] (PE-Cy7). Solid lone : primary antibody Dashed line : isotype control antibody amount : 0.125 microg (5 microl)](https://www.genetex.com/upload/website/prouct_img/normal/GTX01489-10/GTX01489-10_20200428_FACS152_w_23053121_674.webp)
FACS analysis of human peripheral blood monocytes using GTX01489-10 HLA-DR antibody [LN3] (PE-Cy7). Solid lone : primary antibody Dashed line : isotype control antibody amount : 0.125 microg (5 microl)
HLA-DR antibody [LN3] (PE-Cy7)
GTX01489-10
ApplicationsFlow Cytometry
Product group Antibodies
ReactivityHuman
TargetHLA-DRA
Overview
- SupplierGeneTex
- Product NameHLA-DR antibody [LN3] (PE-Cy7)
- Delivery Days Customer9
- Application Supplier NoteFACS: 0.125 microg (5 microl) for 105-108 cells in 100 microl sample per test. *Optimal dilutions/concentrations should be determined by the researcher.Not tested in other applications.
- ApplicationsFlow Cytometry
- CertificationResearch Use Only
- ClonalityMonoclonal
- Clone IDLN3
- Concentration0.025 mg/ml
- ConjugateDouble Conjugated
- Gene ID3122
- Target nameHLA-DRA
- Target descriptionmajor histocompatibility complex, class II, DR alpha
- Target synonymsHLA-DRA1, HLA class II histocompatibility antigen, DR alpha chain, MHC class II antigen DRA, histocompatibility antigen HLA-DR alpha
- HostMouse
- IsotypeIgG2b
- Protein IDP01903
- Protein NameHLA class II histocompatibility antigen, DR alpha chain
- ReactivityHuman
- Storage Instruction2°C to 8°C
- UNSPSC12352203
References
- Eckert F, Schmid U. Identification of plasmacytoid T cells in lymphoid hyperplasia of the skin. Arch Dermatol. 1989,125(11):1518-24.Read this paper






![FACS analysis of human peripheral blood lymphocytes using GTX01489-06 HLA-DR antibody [LN3] (FITC). Solid lone : primary antibody Dashed line : isotype control antibody amount : 0.25 microg (5 microl)](https://www.genetex.com/upload/website/prouct_img/normal/GTX01489-06/GTX01489-06_20200428_FACS90_w_23053121_951.webp)