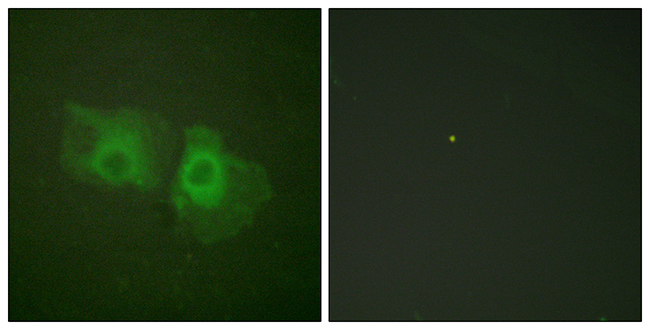
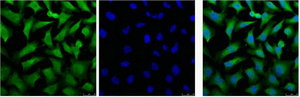
Mouse anti Cytokeratin 18 / Keratin K18
MUB0326P
ApplicationsFlow Cytometry, Western Blot, ImmunoCytoChemistry, ImmunoHistoChemistry, ImmunoHistoChemistry Frozen
Product group Antibodies
ReactivityCanine, Chicken, Hamster, Human, Mouse, Porcine, Rabbit, Rat, Zebra Fish
TargetKRT18
Overview
- SupplierNordic-MUbio
- Product NameMouse anti Cytokeratin 18 / Keratin K18
- Delivery Days Customer7
- Application Supplier NoteRGE53 is suitable for immunoblotting, immunocytochemistry on acetone fixed cells, immunohistochemistry on frozen sections and flow cytometry. Optimal antibody dilution should be determined by titration; recommended range is 1:100 - 1:200 for immunohistochemistry with avidin-biotinylated Horseradish peroxidase complex (ABC) as detection reagent, and 1:100 - 1:1000 for immunoblotting applications.
- ApplicationsFlow Cytometry, Western Blot, ImmunoCytoChemistry, ImmunoHistoChemistry, ImmunoHistoChemistry Frozen
- Applications SupplierFlow Cytometry;Immunocytochemistry;Immunohistochemistry (frozen);Western Blotting
- CertificationResearch Use Only
- ClonalityMonoclonal
- Clone IDRGE53
- Gene ID3875
- Target nameKRT18
- Target descriptionkeratin 18
- Target synonymsCK-18, CYK18, K18, keratin, type I cytoskeletal 18, cell proliferation-inducing gene 46 protein, cytokeratin 18, keratin 18, type I
- HostMouse
- IsotypeIgG1
- Protein IDP05783
- Protein NameKeratin, type I cytoskeletal 18
- SourceRGE53 is a Mouse monoclonal IgG1 antibody derived by fusion of SP2/0-Ag14 mouse myeloma cells with spleen cells from a BALB/c mouse immunized with a cytoskeletal preparation of HeLa cells.
- ReactivityCanine, Chicken, Hamster, Human, Mouse, Porcine, Rabbit, Rat, Zebra Fish
- Reactivity SupplierCanine;Chicken;Hamster;Human;Mouse;Rabbit;Rat;Swine;Zebrafish
- UNSPSC12352203