Mouse anti Human Matrix Metalloproteinase 2 (MMP2)
X2054M
ApplicationsWestern Blot, ELISA, ImmunoHistoChemistry
Product group Antibodies
ReactivityHuman
TargetMMP2
Overview
- SupplierNordic-MUbio
- Product NameMouse anti Human Matrix Metalloproteinase 2 (MMP2)
- Delivery Days Customer7
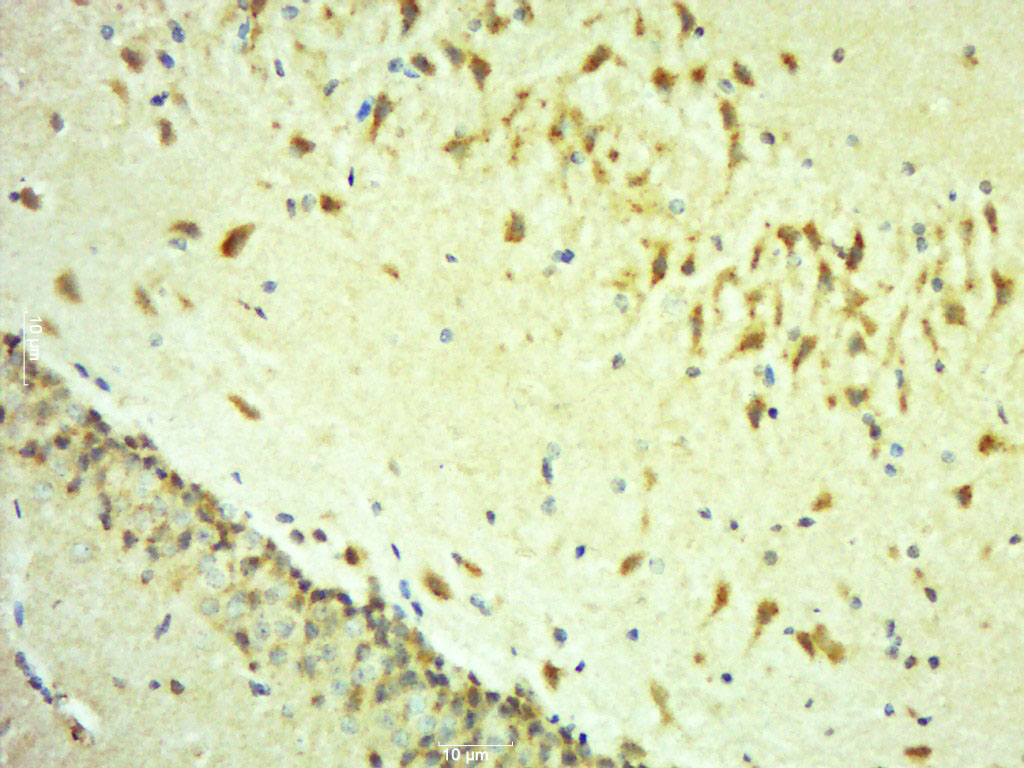
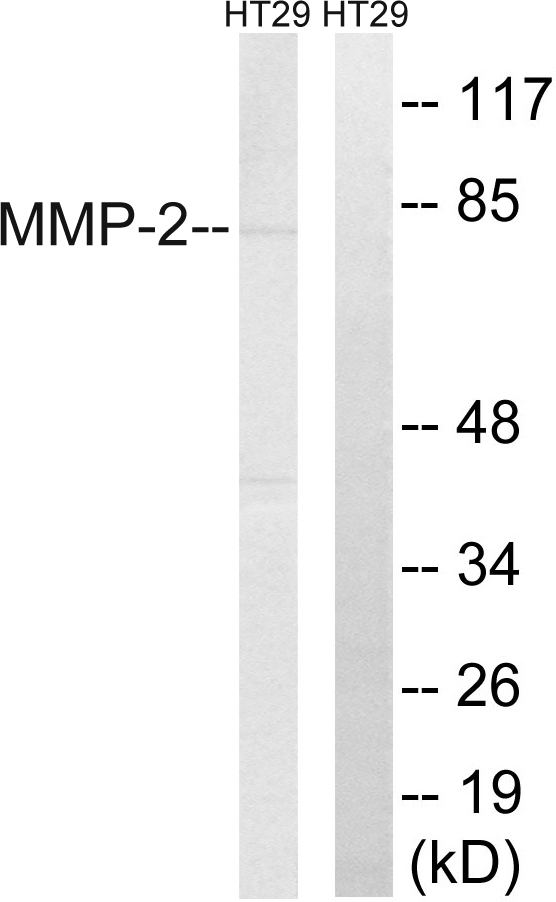
- Application Supplier NoteAntibody can be used for Western blotting (1-2 microg/ml) and immunohistochemistry on formlin-fixed paraffin-embedded tissue sections (1-5 microg/ml). Optimal concentration should be evaluated by serial dilutions.
- ApplicationsWestern Blot, ELISA, ImmunoHistoChemistry
- Applications SupplierWestern Blotting;ELISA;Western Blotting;Immunohistochemistry
- CertificationResearch Use Only
- ClonalityMonoclonal
- Clone ID4D3
- ConjugateUnconjugated
- Gene ID4313
- Target nameMMP2
- Target descriptionmatrix metallopeptidase 2
- Target synonymsCLG4, CLG4A, MMP-2, MMP-II, MONA, TBE-1, 72 kDa type IV collagenase, collagenase type IV-A, matrix metallopeptidase 2 (gelatinase A, 72kDa gelatinase, 72kDa type IV collagenase), matrix metalloproteinase-2, matrix metalloproteinase-II, neutrophil gelatinase
- HostMouse
- IsotypeIgG1
- Protein IDP08253
- Protein Name72 kDa type IV collagenase
- Scientific DescriptionMMP2; MMP-2; CLG4; CLG4A; Matrix metallopeptidase 2; 72 kDa Type IV collagenase precursor; 72 kDa gelatinase, Gelatinase A
- Shelf life instructionSee expiration date on vial
- ReactivityHuman
- Reactivity SupplierHuman
- Reactivity Supplier NoteHybridoma produced by the fusion of splenocytes from BALB/c mice immunized with a synthetic peptide derived from the C-terminus of the human MMP2 protein and mouse myeloma Ag8563 cells.
- UNSPSC12352203