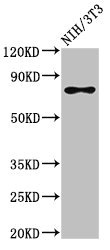
SFPQ antibody [B92]
GTX11825
ApplicationsImmunoFluorescence, ImmunoPrecipitation, Western Blot, ImmunoCytoChemistry, ImmunoHistoChemistry, ImmunoHistoChemistry Frozen, RadioImmunoAssay
Product group Antibodies
ReactivityHuman, Mouse
TargetSFPQ
Overview
- SupplierGeneTex
- Product NameSFPQ antibody [B92]
- Delivery Days Customer9
- Application Supplier NoteWB: 2-4 microg/ml. *Optimal dilutions/concentrations should be determined by the researcher.Not tested in other applications.
- ApplicationsImmunoFluorescence, ImmunoPrecipitation, Western Blot, ImmunoCytoChemistry, ImmunoHistoChemistry, ImmunoHistoChemistry Frozen, RadioImmunoAssay
- CertificationResearch Use Only
- ClonalityMonoclonal
- Clone IDB92
- Concentration2 mg/ml
- ConjugateUnconjugated
- Gene ID6421
- Target nameSFPQ
- Target descriptionsplicing factor proline and glutamine rich
- Target synonymsPOMP100, PPP1R140, PSF, splicing factor, proline- and glutamine-rich, 100 kDa DNA-pairing protein, DNA-binding p52/p100 complex, 100 kDa subunit, PTB-associated splicing factor, epididymis secretory sperm binding protein, polypyrimidine tract binding protein associated, polypyrimidine tract-binding protein-associated splicing factor, protein phosphatase 1, regulatory subunit 140, splicing factor proline/glutamine rich (polypyrimidine tract binding protein associated), splicing factor proline/glutamine-rich
- HostMouse
- IsotypeIgG1
- Protein IDP23246
- Protein NameSplicing factor, proline- and glutamine-rich
- Scientific DescriptionThe RNAs that direct protein synthesis in animals and plant cells are synthesized in the nucleus as large precursors (pre-mRNAs). The protein coding sequences in pre-mRNA molecules are arranged in discontinuous segments - exons interspersed with noncoding sequences - introns. In a process termed splicing, these introns are efficiently removed before the pre-mRNA is transported from the nucleus to the cytoplasm, where it is translated into protein. Studies have shown that nuclear pre-mRNA splicing takes place in a multi-component structure termed a spliceosome. The polypyrimidine tract-binding (PTB) protein-associated splicing factor (PSF), which plays an essential role in mammalian spliceosomes, is a ubiquitous nuclear matrix protein. A complex between PTB and PSF is necessary for pre-mRNA splicing. PSF contains two consensus RNA-binding domains and an unusual amino terminus rich in proline and glutamine residues. The RNA-binding properties of PSF are apparently identical to those of PTB. Both proteins, together and independently, bind the polypyrimidine tract of mammalian introns. However, the nuclear localization of PSF and PTB and their distribution in subnuclear fractions differ markedly: isolated nuclear matrices contain a bulk of PSF, but only minor amounts of PTB. In confocal microscopy both proteins appear in speckles, the majority of which do not co-localize. These PTB/PSF complexes, as well as the observed PSF-PTB interaction, may reflect the presence of PTB and PSF in spliceosomal complexes during RNA processing, although other data point to different cellular distribution and nuclear matrix association of the majority of PSF and PTB. The cleavage of PSF during lysis of immature myeloid cells is accompanied by digestion of the PTB splicing regulator but not other proteins tested. In contrast, during apoptosis PTB is degraded while PSF remains intact. Proteolytic degradation of PSF specifically occurs in intact myeloid cells and this process is enhanced upon immature myeloid cell lysis; PSF is completely cleaved to a 47 kDa proteolytic cleavage product (p47), due to potent proteolytic activity found in these cells but rare in other cells and tissues. Furthermore, p47 is abundant in intact normal and tumor myeloid cells while in other cell types it is undetectable. The bone marrow 47 kDa protein is a fragment constituting the N-terminal, protease-resistant half of the splicing factor PSF. PSF is highly basic and migrates anomalously on SDS gels. The 47 kDa protein of mouse cells of immature myeloid origin (bone marrow and acute myeloid leukemia) exhibits a gel migration pattern corresponding to a 49 kDa molecule. In other cell types such as lymphoid cells and in peripheral blood cells, PSF appears as approx. 100 kDa or 75 kDa molecules. The sequence of a fragment of mouse PSF was found to be remarkably similar to that of human PSF (> 98% homology). Also, the sequences of PSF and the human (h) 100 kDa DNA-pairing protein (hPOMp100) reveals identity. Homologous pairing is a fundamental biological reaction implicated in various cellular processes such as DNA recombination and repair, chromosome pairing, sister chromatid cohesion and chromosome condensation, gene inactivation and initiation of replication. The base pairing is also involved in spliceosome assembly resulting in formation of a dynamic Holliday-like structure within which splicing occurs. Indeed, PSF/hPOMp100 bind both singlestranded (ss) and double-stranded (ds) DNA and facilitates the renaturation of complementary ssDNA molecules. Importantly, PSF/hPOMp100 promotes the formation of D-loops in superhelical duplex DNA. PSF/hPOMp100 also serves as an efficient substrate for protein kinase C (PKC) in vitro. PKC phosphorylation of PSF/hPOMp100 stimulates its DNA binding and D-loop formation activity suggesting a possible regulatory mechanism. PSF has been demonstrated to interact with a variety of cellular targets including the human pro-oncoproteins EWS, hPOMp75/TLS and calmodulin, the RNA/DNAbinding nuclear protein p54nrb/NonO (the homolog of PSF) and DNA topoisomerase. A direct interaction has been observed, between PSF and topoisomerase I which has been implied in DNA recombination, DNA repair, and chromosome formation and may act as a transcription factor and a protein kinase. PSF is also expressed by differentiating neurons in developing mouse brain. Both the expression of PSF mRNA in cortex and cerebellum and PSF immunoreactivity in all brain areas has been found to be high during embryonic and early postnatal life. In adult tissue, only various neuronal populations in the hippocampus and olfactory bulb express PSF. PSF is expressed by differentiating neurons but not by astrocytic cells including radial glia; however oligodendrocytes differentiating in vitro were found to express it. The restricted expression of PSF suggests that it is involved in the control of neuronal-specific splicing events occurring at particular stages of neuronal differentiation and maturation. Monoclonal antibodies reacting specifically with PSF are useful tools for the molecular identification and characterization of the functional activity of PSF.
- ReactivityHuman, Mouse
- Storage Instruction-20°C or -80°C,2°C to 8°C
- UNSPSC12352203