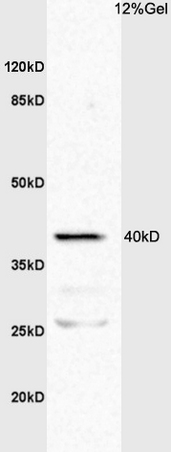
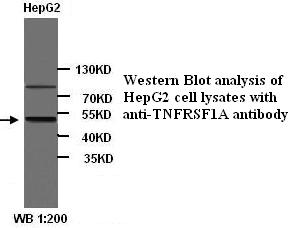
TNF Receptor I antibody
GTX10500
ApplicationsWestern Blot, ELISA
Product group Antibodies
ReactivityHuman, Mouse
TargetTNFRSF1A
Overview
- SupplierGeneTex
- Product NameTNF Receptor I antibody
- Delivery Days Customer9
- ApplicationsWestern Blot, ELISA
- CertificationResearch Use Only
- ClonalityPolyclonal
- ConjugateUnconjugated
- Gene ID7132
- Target nameTNFRSF1A
- Target descriptionTNF receptor superfamily member 1A
- Target synonymsCD120a, FPF, TBP1, TNF-R, TNF-R-I, TNF-R55, TNFAR, TNFR1, TNFR55, TNFR60, p55, p55-R, p60, tumor necrosis factor receptor superfamily member 1A, TNF-R1, TNF-RI, TNFR-I, tumor necrosis factor binding protein 1, tumor necrosis factor receptor type 1, tumor necrosis factor-alpha receptor
- HostGoat
- IsotypeIgG
- Protein IDP19438
- Protein NameTumor necrosis factor receptor superfamily member 1A
- Scientific DescriptionTNF RI (CD120a) is a 55 kDa transmembrane glycoprotein that is expressed by virtually all nucleated mammalian cells. Among the numerous cells known to express TNF RI are hepatocytes, monocytes and neutrophils, cardiac muscle cells, endothelial cells, and CD34+ hematopoietic progenitors. Both TNF-alpha and TNF-beta bind to TNF RI. Soluble TNF-alpha binds with a Kda in the range of 20-60 pM, while TNF-beta binds with a Kda equal to 650 pM. TNF RI relative to TNF RII seems to be the more physiologically-relevant receptor, whereas TNF-R2 appears to play a direct role in only a limited number of TNF responses. Soluble TNF RI, which blocks TNF-alpha activity, has been identified in both urine and blood (1-3 ng/mL). Serum levels of sTNF receptors increase dramatically in certain pathological situations. Two soluble forms have been identified and are believed to be generated by proteolytic cleavage. Human TNF RI has 64% amino acid sequence identity (70% in the extracellular region) with mouseTNF-R1, and human TNF RI binds human and mouse TNF-alpha with equal affinity. The extracellular region has four cysteine-rich motifs, the first of which is suggested to be required for binding. The intracellular portion of TNF R1 contains a death domain of about 70 amino acids that is required for the signaling of apoptosis and NF-kappaB activation. TNF binds to the extracellular domain of TNF R1 and induces receptor trimerization. Then, the aggregated death domain of TNF R1 recruits the adapter protein TRADD. TRADD, in turn, recruits FADD, TRAF2 and RIP to form the TNF R1 signaling complex and activate signaling cascades leading to apoptosis, JNK/SAPK activation, and NF-kappaB activation respectively. However, TNF R1 self-associates and signals independently of ligand when overexpressed. This apparent paradox may be explained by silencer of death domains (SODD), a widely expressed approximately 60 kDa protein that was found to be associated with the death domain of TNF-RI.
- ReactivityHuman, Mouse
- Storage Instruction-20°C or -80°C,2°C to 8°C
- UNSPSC12352203