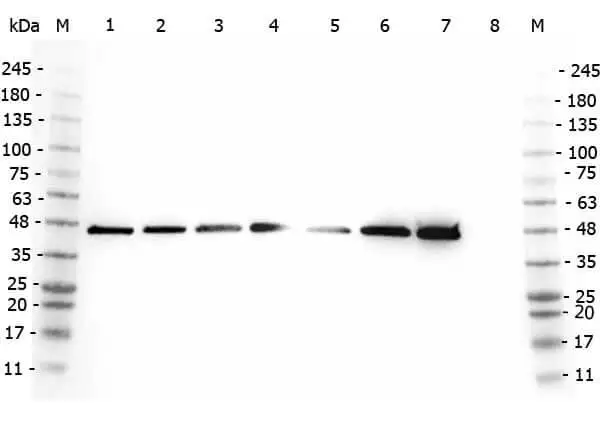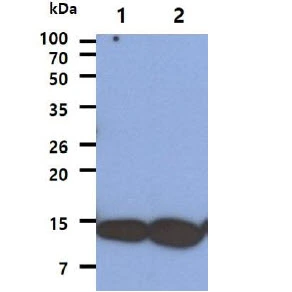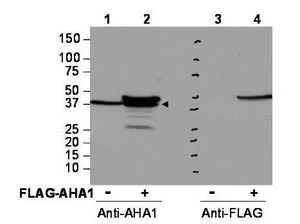
Western blot using GeneTex's affinity purified anti-AHA1 antibody shows detection of AHA1 in Cos7 cells. For Lanes 2 and 4, Cos7 cells were transfected with pcDNA3-FLAG-AHA1. For Lanes 1 and 3, Cos7 cells were not transfected. Extracts (40 μg per lane) were electrophoresed and transferred to nitrocellulose. The membrane was probed with anti-AHA1 (lanes 1 and 2, 1:2,000 dilution) or anti-FLAG (lanes 3 and 4). The lower band seen in anti-AHA1 blotting (arrowhead) is endogenous AHA1.
AHA-1 antibody
GTX48723
ApplicationsImmunoPrecipitation, Western Blot, ELISA, ImmunoHistoChemistry
Product group Antibodies
ReactivityHuman, Monkey
TargetAHSA1
Overview
- SupplierGeneTex
- Product NameAHA-1 antibody
- Delivery Days Customer9
- Application Supplier NoteWB: 1 microg/mL. ELISA: 1:35000-1:185000. *Optimal dilutions/concentrations should be determined by the researcher.Not tested in other applications.
- ApplicationsImmunoPrecipitation, Western Blot, ELISA, ImmunoHistoChemistry
- CertificationResearch Use Only
- ClonalityPolyclonal
- Concentration1 mg/ml
- ConjugateUnconjugated
- Gene ID10598
- Target nameAHSA1
- Target descriptionactivator of HSP90 ATPase activity 1
- Target synonymsAHA1, C14orf3, hAha1, p38, activator of 90 kDa heat shock protein ATPase homolog 1, AHA1, activator of heat shock 90kDa protein ATPase homolog 1
- HostRabbit
- IsotypeIgG
- Protein IDO95433
- Protein NameActivator of 90 kDa heat shock protein ATPase homolog 1
- Scientific DescriptionActivator of Hsp90 ATPase (AHA1) stimulates the inherent ATPase cycle of Hsp90, which is essential for its chaperone activity in vivo. The activation and/or stability of many of the key regulatory and signaling proteins of the eukaryotic cell depend on their interaction with the Hsp90 molecular chaperone. Hsp90 is assisted and regulated by co-chaperones that participate in an ordered series both to assist client-protein recruitment or release and to modulate progress through the ATPase coupled chaperone cycle. Structural analysis and mutagenesis show that binding of the N-terminal domain of AHA1 to Hsp90 promotes a conformational switch in the middle-segment catalytic loop (aa 370-390) of Hsp90 that exposes the catalytic Arg380 and enables its interaction with ATP in the N-terminal nucleotide-binding domain of the chaperone. Recent studies show that AHA1 modulates Hsp90-dependent stability of the folding of the cystic fibrosis transmembrane conductance regulator (CFTR) in the endoplasmic reticulum (ER). Down-regulation of AHA1 rescues misfolding of CFTR in cystic fibrosis.
- ReactivityHuman, Monkey
- Storage Instruction-20°C or -80°C,2°C to 8°C
- UNSPSC41116161