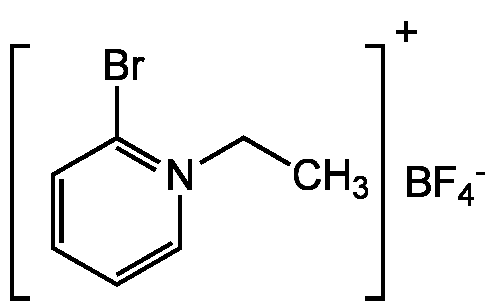
Chemical Structure
BEP [878-23-9] [878-23-9]
CDX-B0040
CAS Number878-23-9
Product group Chemicals
Estimated Purity>97%
Molecular Weight273.86
Overview
- SupplierChemodex
- Product NameBEP [878-23-9] [878-23-9]
- Delivery Days Customer2
- CAS Number878-23-9
- CertificationResearch Use Only
- Estimated Purity>97%
- Molecular FormulaC7H9BBrF4N
- Molecular Weight273.86
- Scientific DescriptionChemical. CAS: 878-23-9. Formula: C7H9BBrF4N. MW: 273.86. Synthetic Efficient coupling reagent for the synthesis of sterically hindered amides, especially peptides. In terms of reactivity and racemization-suppressing capability, this pyridinium-type reagent, BEP, proved to be more efficient than commonly used uronium- and phosphonium-type reagents, such as HBTU, BOP, and PyBroP, for the synthesis of hindered peptides containing N-methylated or Calpha,Calpha-dialkylated amino acid residues. BEP was also proven to be an efficient reagent for the synthesis of esters, especially active esters and hindered esters, and tert-butyl esters. - Efficient coupling reagent for the synthesis of sterically hindered amides, especially peptides. In terms of reactivity and racemization-suppressing capability, this pyridinium-type reagent, BEP, proved to be more efficient than commonly used uronium- and phosphonium-type reagents, such as HBTU, BOP, and PyBroP, for the synthesis of hindered peptides containing N-methylated or Calpha,Calpha-dialkylated amino acid residues. BEP was also proven to be an efficient reagent for the synthesis of esters, especially active esters and hindered esters, and tert-butyl esters.
- SMILES[F-].FB(F)F.CC[N]1=C(Br)C=CC=C1
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200