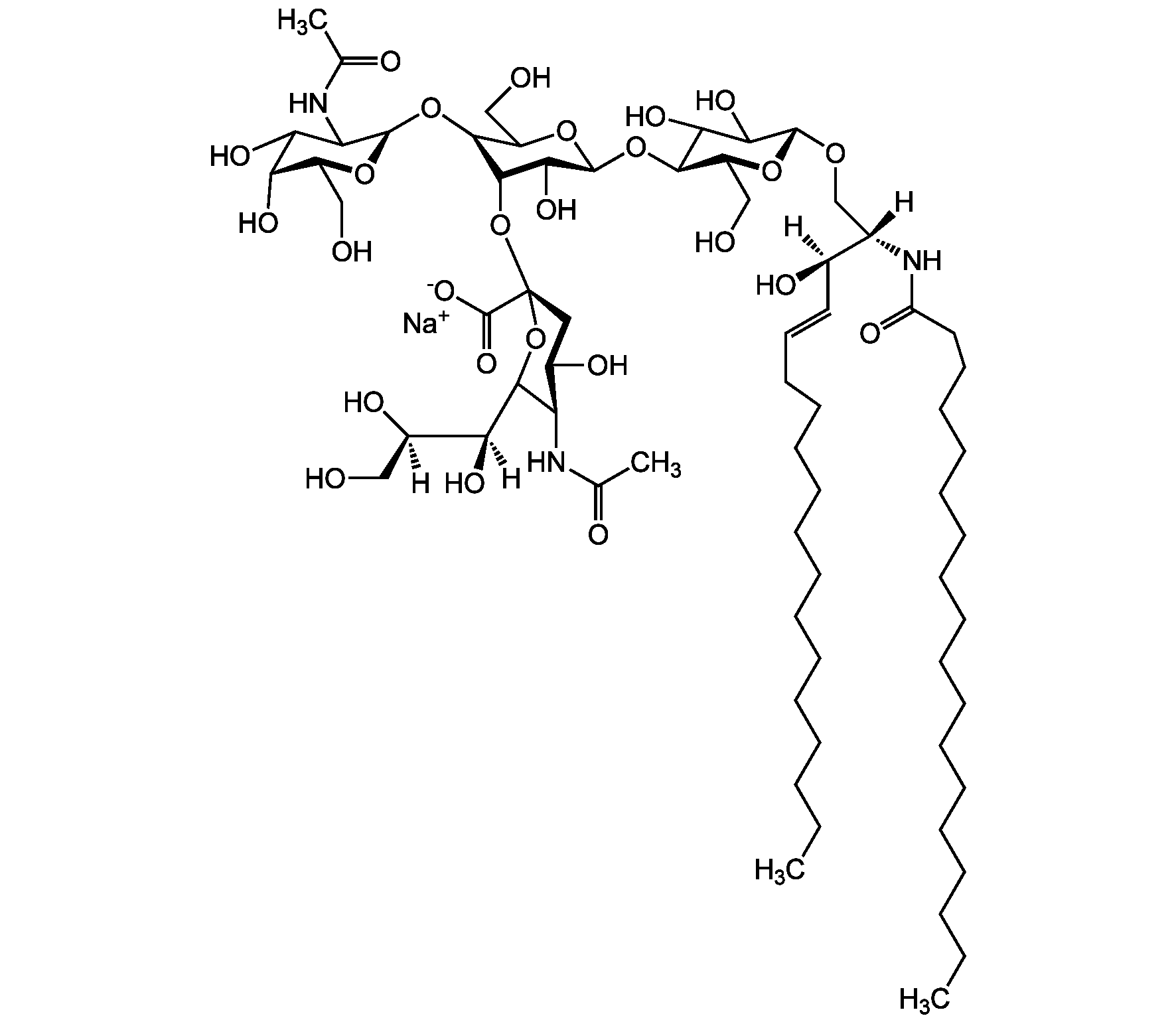
Chemical Structure
Ganglioside GD1a . disodium salt (bovine brain) [12707-58-3] [12707-58-3]
AG-CN2-9003
CAS Number12707-58-3
Product group Chemicals
Estimated Purity>98%
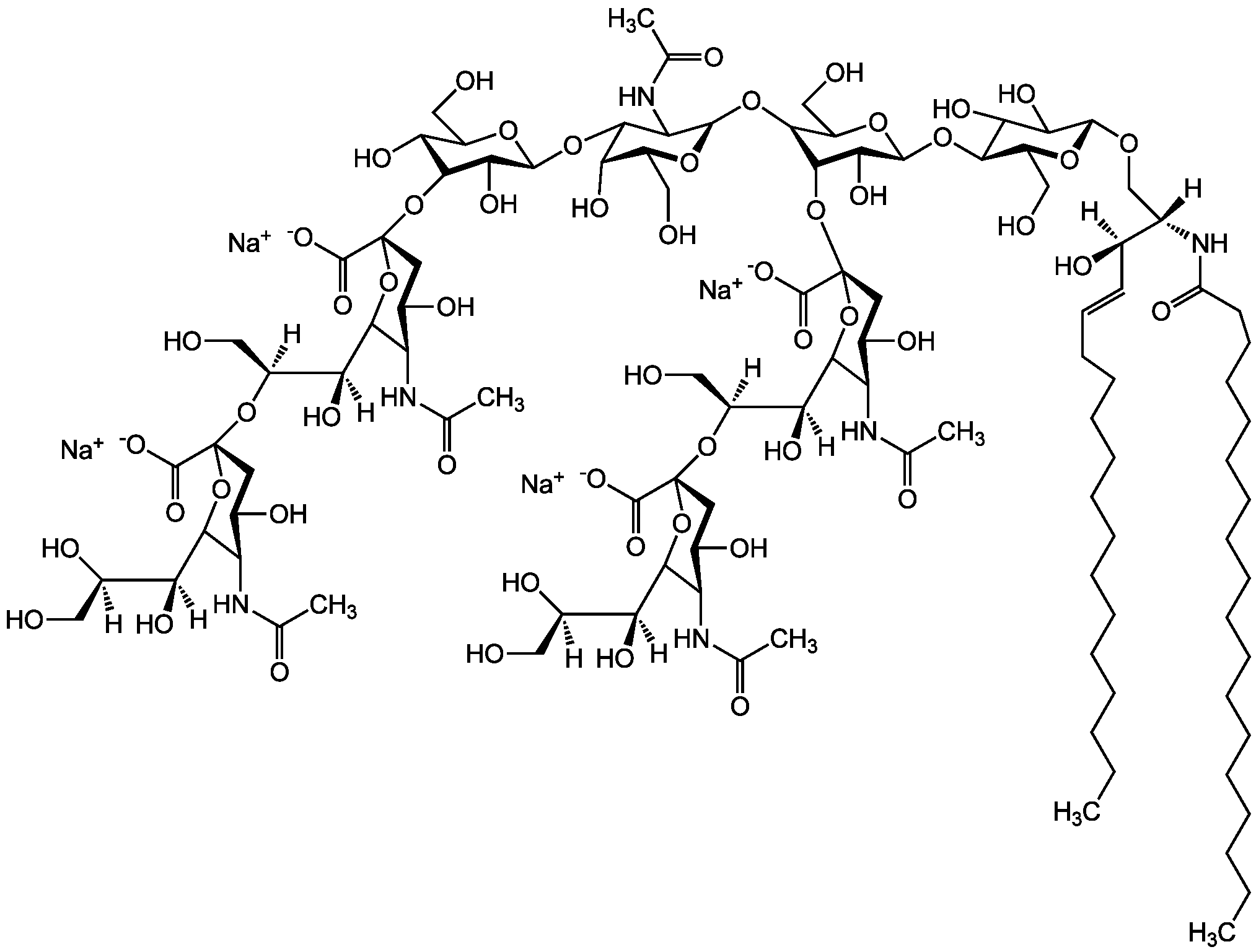
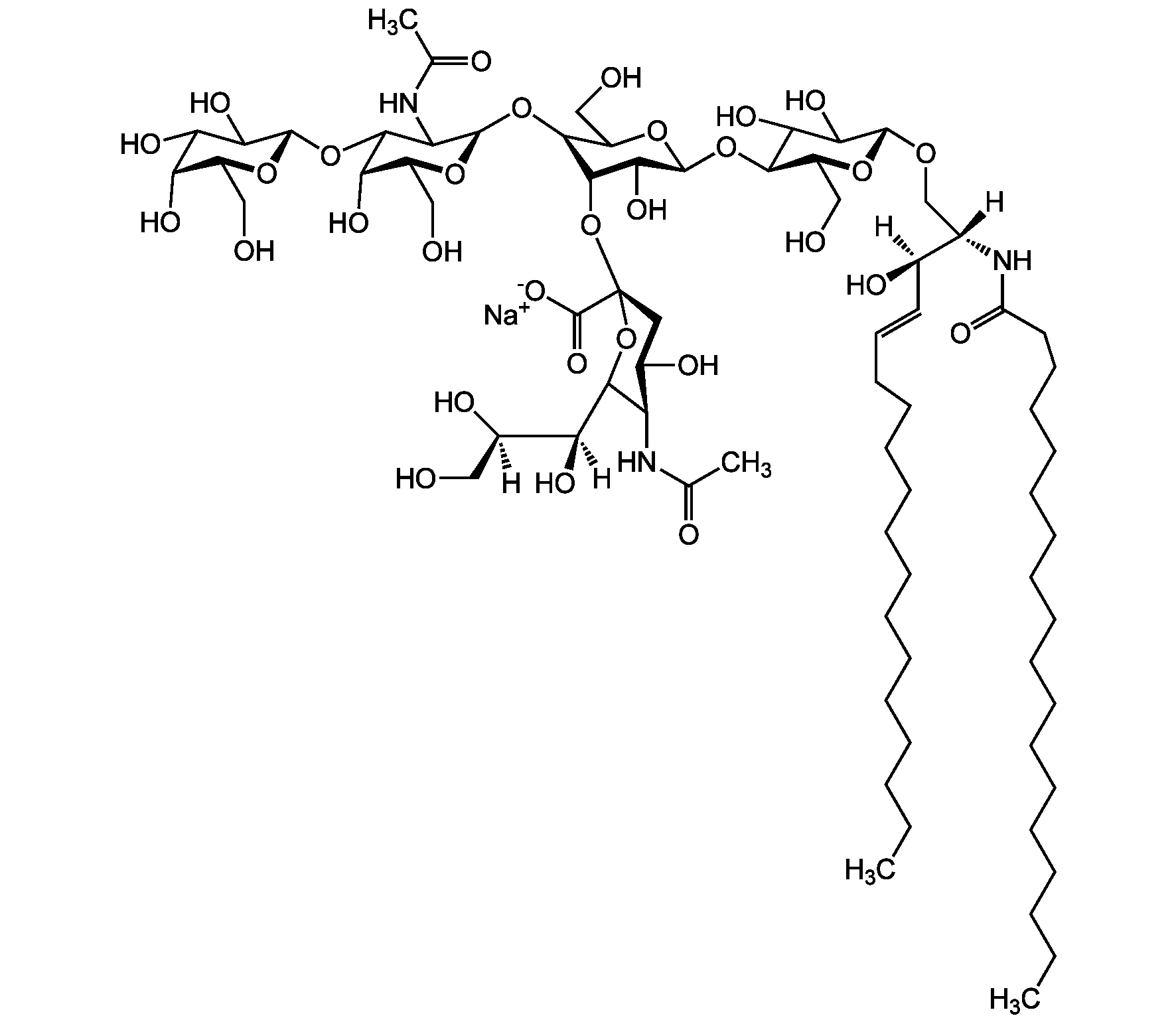
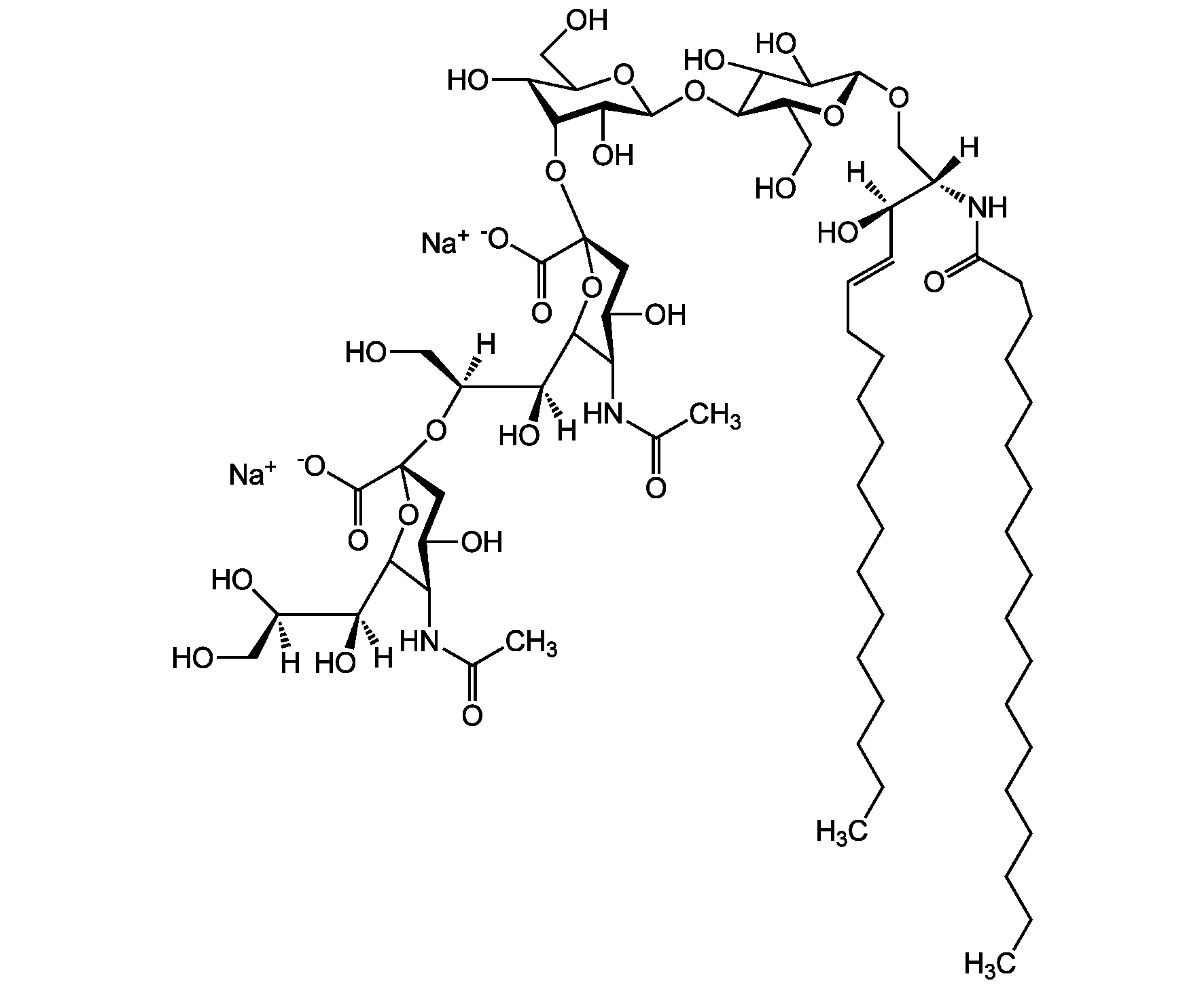
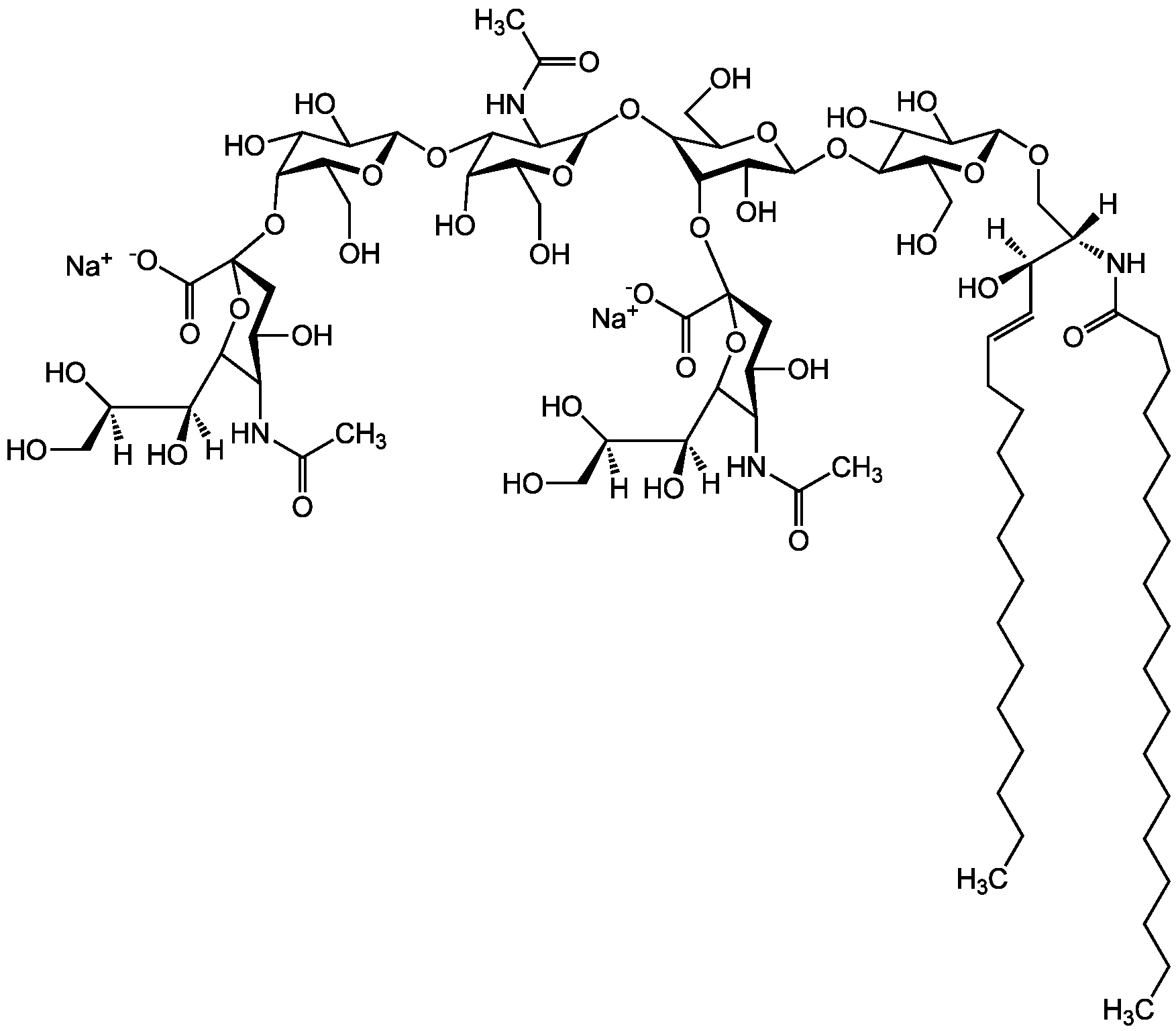
Molecular Weight1836.1 . 46.0 (calculated on sphingosine C18:1 and stearic acid)
Overview
- SupplierAdipoGen Life Sciences
- Product NameGanglioside GD1a . disodium salt (bovine brain) [12707-58-3] [12707-58-3]
- Delivery Days Customer10
- CAS Number12707-58-3
- CertificationResearch Use Only
- Estimated Purity>98%
- Molecular FormulaC84H146N4O39 . 2Na
- Molecular Weight1836.1 . 46.0 (calculated on sphingosine C18:1 and stearic acid)
- Scientific DescriptionChemical. CAS: 12707-58-3. Formula: C84H146N4O39 . 2Na. MW: 1836.1 . 46.0 (calculated on sphingosine C18:1 and stearic acid). Isolated from bovine brain. Gangliosides are acidic glycosphingolipids that form lipid rafts in the outer leaflet of the cell plasma membrane, especially in neuronal cells in the central nervous system. They participate in cellular proliferation, differentiation, adhesion, signal transduction, cell-to-cell interactions, tumorigenesis and metastasis. The accumulation of gangliosides has been linked to several diseases. Ganglioside GD1a is the major ganglioside of the nervous system. It is converted to GM1 by bacterial, viral and mammalian sialidases. It is a differentiation marker for cell growth. - Gangliosides are acidic glycosphingolipids that form lipid rafts in the outer leaflet of the cell plasma membrane, especially in neuronal cells in the central nervous system. They participate in cellular proliferation, differentiation, adhesion, signal transduction, cell-to-cell interactions, tumorigenesis and metastasis. The accumulation of gangliosides has been linked to several diseases. Ganglioside GD1a is the major ganglioside of the nervous system. It is converted to GM1 by bacterial, viral and mammalian sialidases. It is a differentiation marker for cell growth.
- SMILES[Na+].[Na+].[H][C@@](O)(CO)[C@]([H])(O)C1O[C@@](CC(O)[C@H]1NC(C)=O)(O[C@@H]1C(O)C(O)[C@H](OC2[C@@H](O)[C@H](CO)O[C@H](O[C@H]3C(CO)O[C@@H](O[C@H]4C(O)C(O)[C@H](OC[C@]([H])(NC(=O)CCCCCCCCCCCCCCCCC)[C@]([H])(O)\C=C\CCCCCCCCCCCCC)O[C@H]4CO)C(O)[C@H]3O[C@@]3(CC(O)[C@@H](NC(C)=O)C(O3)[C@@]([H])(O)[C@]([H])(O)CO)C([O-])=O)C2NC(C)=O)O[C@H]1CO)C([O-])=O
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200