K11-linked Tetra-Ubiquitin (human) (rec.) (untagged)
SBB-UP0064
Protein IDP0CG47
Product group Proteins / Signaling Molecules
Overview
- SupplierSouth Bay Bio
- Product NameK11-linked Tetra-Ubiquitin (human) (rec.) (untagged)
- Delivery Days Customer10
- CertificationResearch Use Only
- Estimated Purity>95%
- Gene ID7314
- Target nameUBB
- Target descriptionubiquitin B
- Target synonymsHEL-S-50, polyubiquitin-B, epididymis secretory protein Li 50, polyubiquitin B
- Protein IDP0CG47
- Protein NamePolyubiquitin-B
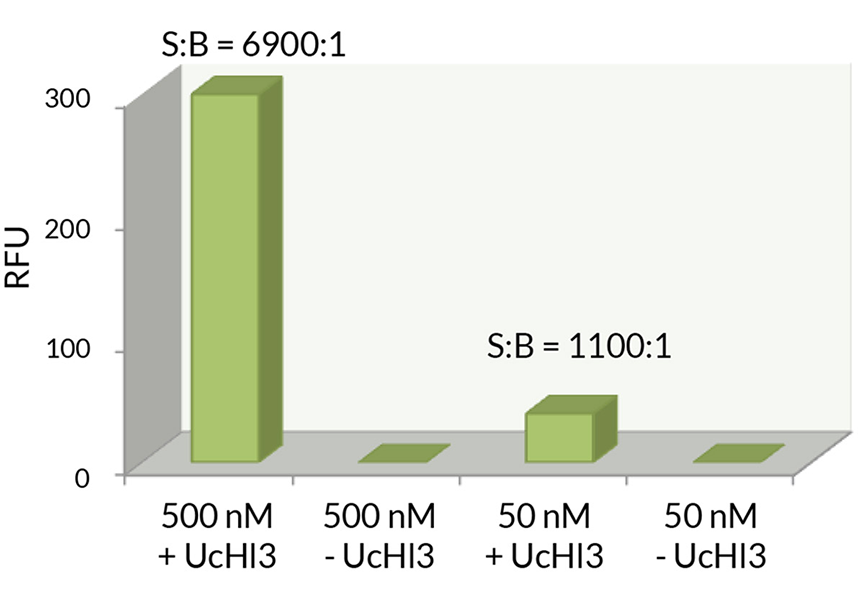
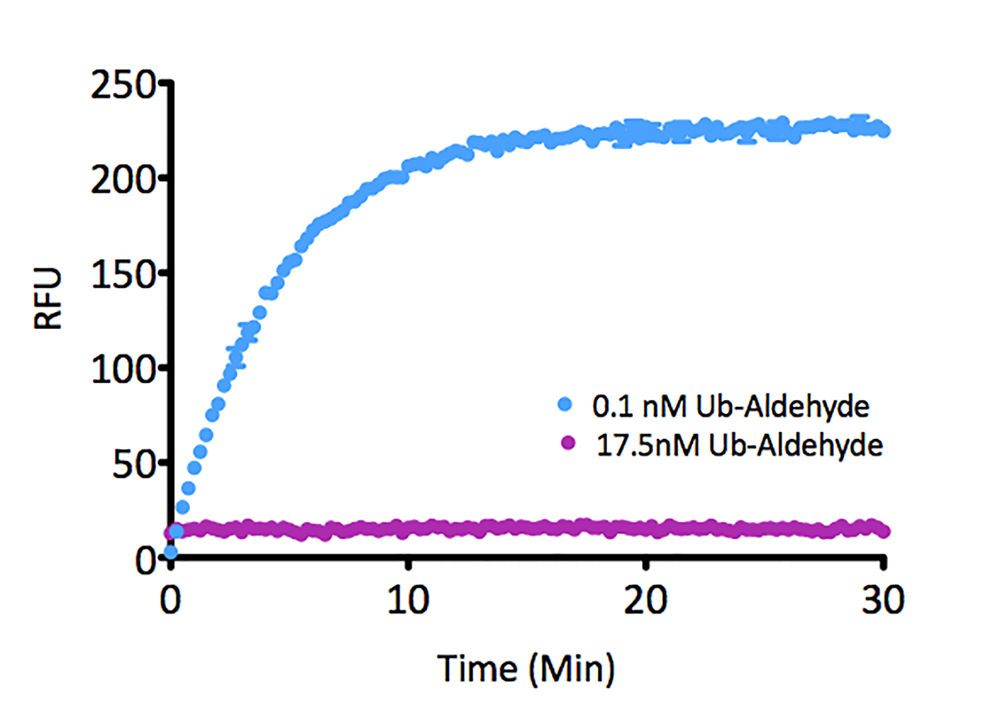
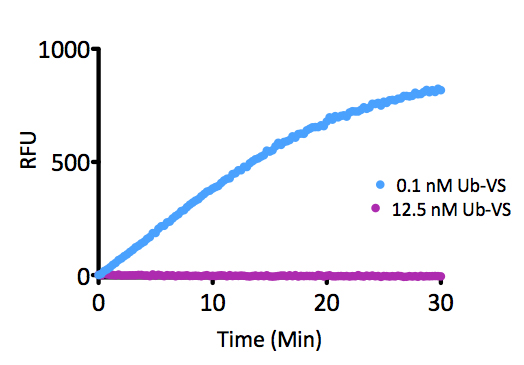
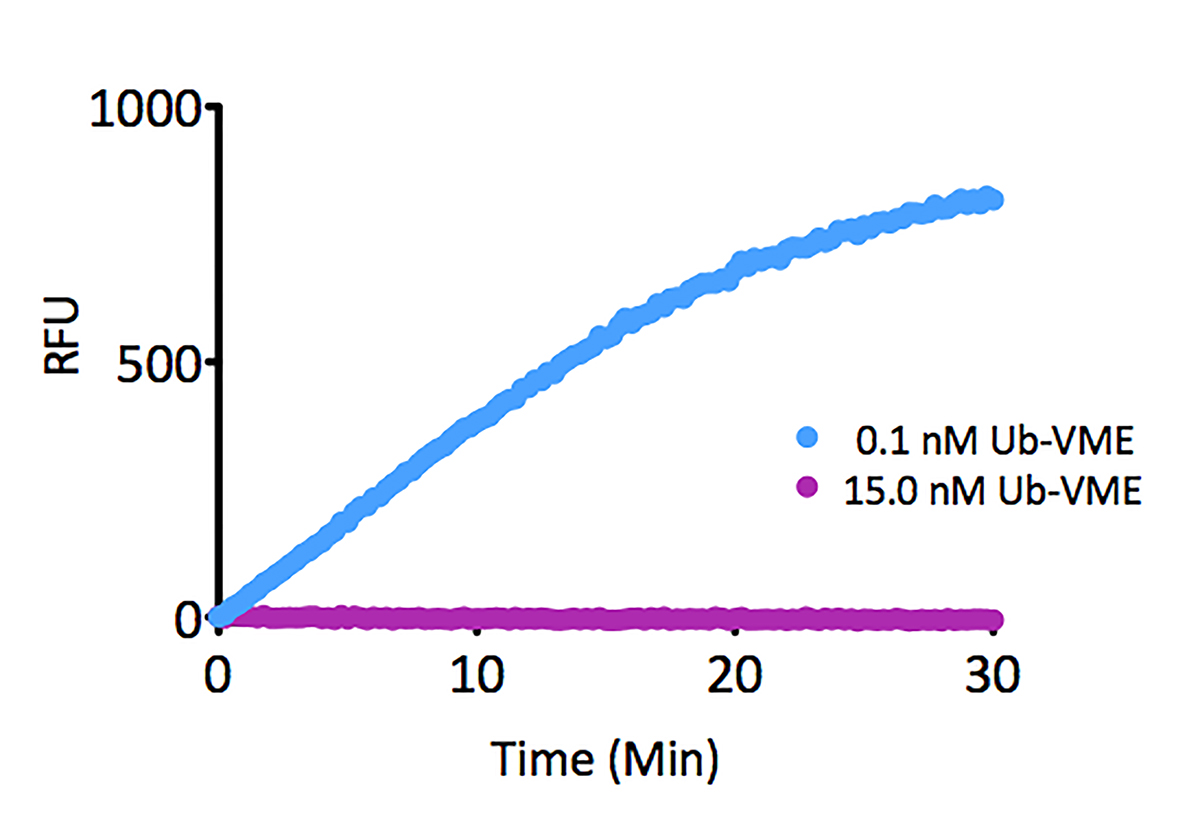
- Scientific DescriptionRecombinant Protein. Four enzymatically conjugated human ubiquitin (aa1-76) covalently linked through an isopeptide bond at K11 residue of one ubiquitin molecule and the C-terminal glycine residue of another ubiquitin molecule and untagged. Source/Host: E. coli. Liquid. In 50mM HEPES pH 7.5. The array of cellular processes initiated and regulated by ubiquitin has been partially explained by the structural diversity of differently linked ubiquitin chains. In a ubiquitin chain, ubiquitin moieties can be conjugated through one of their lysine residues (K6, K11, K27, K29, K33, K48 and K63) or the N-terminal methionine residue (M1), offering countless possibilities to assemble a specific polymer. Ubiquitin molecules can also be modified by other post-translational modifications, including acetylation and phosphorylation, adding another layer of ubiquitin signal regulation and diversification. The abundance of K11 linkages strongly increase when the metazoan anaphase-promoting complex APC/C is active during mitosis, and APC/C has been shown to assemble K11-linked ubiquitin chains to drive proteasomal degradation and exit from mitosis. This protein is formed with wild-type human recombinant ubiquitin and linkage-specific enzymes. Ideal for investigating ubiquitin-binding proteins and as substrates for ubiquitin-specific isopeptidases. Reaction conditions will need to be optimized for each specific application. - The array of cellular processes initiated and regulated by ubiquitin has been partially explained by the structural diversity of differently linked ubiquitin chains. In a ubiquitin chain, ubiquitin moieties can be conjugated through one of their lysine residues (K6, K11, K27, K29, K33, K48 and K63) or the N-terminal methionine residue (M1), offering countless possibilities to assemble a specific polymer. Ubiquitin molecules can also be modified by other post-translational modifications, including acetylation and phosphorylation, adding another layer of ubiquitin signal regulation and diversification. The abundance of K11 linkages strongly increase when the metazoan anaphase-promoting complex APC/C is active during mitosis, and APC/C has been shown to assemble K11-linked ubiquitin chains to drive proteasomal degradation and exit from mitosis. This protein is formed with wild-type human recombinant ubiquitin and linkage-specific enzymes. Ideal for investigating ubiquitin-binding proteins and as substrates for ubiquitin-specific isopeptidases. Reaction conditions will need to be optimized for each specific application.
- Storage Instruction-80°C
- UNSPSC41116100
- SpeciesHuman


![Ubiquitin Activating Enzyme E1 [UBA1; UBE1] (human) (rec.) (His)](https://adipogen.com/pub/media/catalog/product/s/b/sbb-ce0058_finalgel.png)