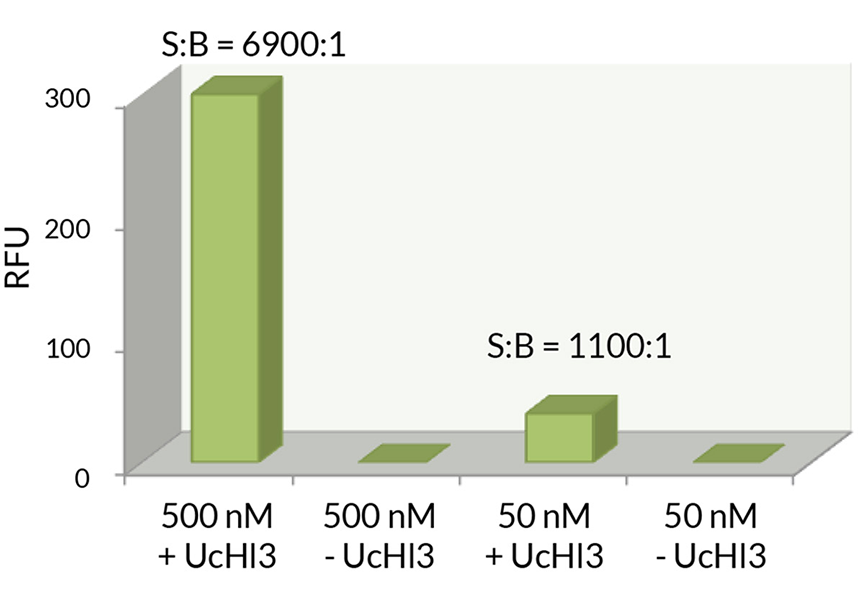
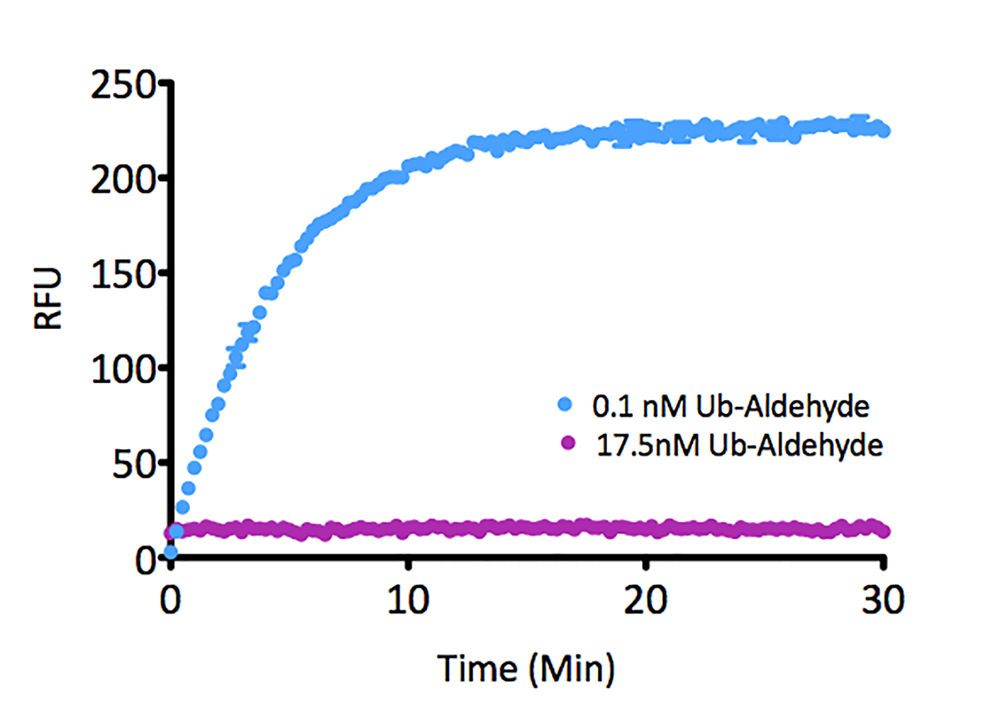
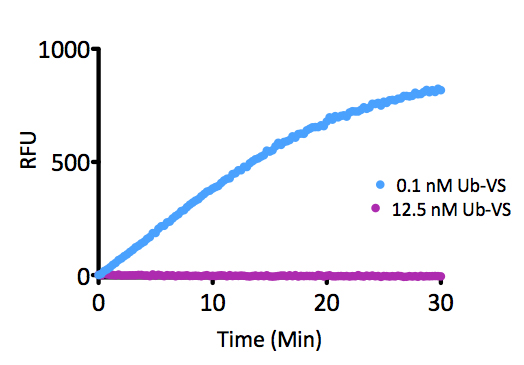
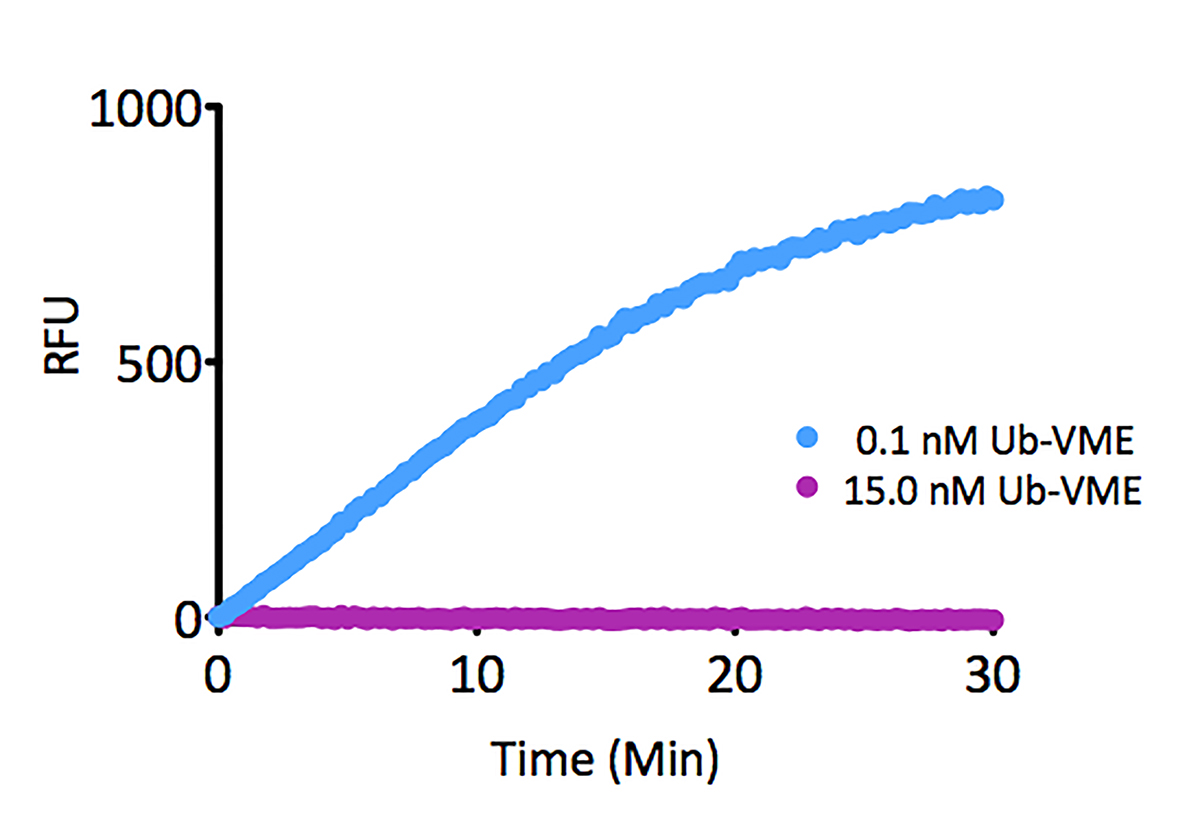
K6-linked Tetra-Ubiquitin (human) (rec.) (untagged)
SBB-UP0061
Protein IDP0CG47
Product group Proteins / Signaling Molecules
Overview
- SupplierSouth Bay Bio
- Product NameK6-linked Tetra-Ubiquitin (human) (rec.) (untagged)
- Delivery Days Customer10
- CertificationResearch Use Only
- Estimated Purity>95%
- Gene ID7314
- Target nameUBB
- Target descriptionubiquitin B
- Target synonymsHEL-S-50, polyubiquitin-B, epididymis secretory protein Li 50, polyubiquitin B
- Protein IDP0CG47
- Protein NamePolyubiquitin-B
- Scientific DescriptionRecombinant Protein. Four enzymatically conjugated human ubiquitin (aa1-76) covalently linked through an isopeptide bond at K6 residue of one ubiquitin molecule and the C-terminal glycine residue of another ubiquitin molecule and untagged. Source/Host: E. coli. Liquid. In 50mM HEPES pH 7.5. The array of cellular processes initiated and regulated by ubiquitin has been partially explained by the structural diversity of differently linked ubiquitin chains. In a ubiquitin chain, ubiquitin moieties can be conjugated through one of their lysine residues (K6, K11, K27, K29, K33, K48 and K63) or the N-terminal methionine residue (M1), offering countless possibilities to assemble a specific polymer. Ubiquitin molecules can also be modified by other post-translational modifications, including acetylation and phosphorylation, adding another layer of ubiquitin signal regulation and diversification. K6-linked ubiquitin linkages are found at increased levels in response to UV radiation, indirectly associated with DNA repair, and have been identified on mitochondrial outer membrane (MOM) proteins. This protein is formed with wild-type human recombinant ubiquitin and linkage-specific enzymes. Ideal for investigating ubiquitin-binding proteins and as substrates for ubiquitin-specific isopeptidases. Reaction conditions will need to be optimized for each specific application. - The array of cellular processes initiated and regulated by ubiquitin has been partially explained by the structural diversity of differently linked ubiquitin chains. In a ubiquitin chain, ubiquitin moieties can be conjugated through one of their lysine residues (K6, K11, K27, K29, K33, K48 and K63) or the N-terminal methionine residue (M1), offering countless possibilities to assemble a specific polymer. Ubiquitin molecules can also be modified by other post-translational modifications, including acetylation and phosphorylation, adding another layer of ubiquitin signal regulation and diversification. K6-linked ubiquitin linkages are found at increased levels in response to UV radiation, indirectly associated with DNA repair, and have been identified on mitochondrial outer membrane (MOM) proteins. This protein is formed with wild-type human recombinant ubiquitin and linkage-specific enzymes. Ideal for investigating ubiquitin-binding proteins and as substrates for ubiquitin-specific isopeptidases. Reaction conditions will need to be optimized for each specific application.
- Storage Instruction-80°C
- UNSPSC41116100
- SpeciesHuman

![Ubiquitin Activating Enzyme E1 [UBA1; UBE1] (human) (rec.) (His)](https://adipogen.com/pub/media/catalog/product/s/b/sbb-ce0058_finalgel.png)