![WB analysis of rabbit tongue extract using GTX11083 Myosin skeletal slow antibody [NOQ7.5.4D].
Lane A: Antibody dilution 1:5,000
Lane B: Secondary antibody only WB analysis of rabbit tongue extract using GTX11083 Myosin skeletal slow antibody [NOQ7.5.4D].
Lane A: Antibody dilution 1:5,000
Lane B: Secondary antibody only](https://www.genetex.com/upload/website/prouct_img/normal/GTX11083/GTX11083_20170605_WB_w_23060500_523.webp)
WB analysis of rabbit tongue extract using GTX11083 Myosin skeletal slow antibody [NOQ7.5.4D].
Lane A: Antibody dilution 1:5,000
Lane B: Secondary antibody only
Myosin skeletal slow antibody [NOQ7.5.4D]
GTX11083
ApplicationsElectron Microscopy, Western Blot, ELISA, ImmunoHistoChemistry, ImmunoHistoChemistry Frozen, ImmunoHistoChemistry Paraffin, RadioImmunoAssay
Product group Antibodies
ReactivityBovine, Canine, Chicken, Feline, Guinea Pig, Goat, Hamster, Human, Mouse, Porcine, Rabbit, Rat, Sheep
TargetMYH7
Overview
- SupplierGeneTex
- Product NameMyosin skeletal slow antibody [NOQ7.5.4D]
- Delivery Days Customer9
- Application Supplier NoteWB: 1:5,000. IHC-P: 1:4,000. *Optimal dilutions/concentrations should be determined by the researcher.Not tested in other applications.
- ApplicationsElectron Microscopy, Western Blot, ELISA, ImmunoHistoChemistry, ImmunoHistoChemistry Frozen, ImmunoHistoChemistry Paraffin, RadioImmunoAssay
- CertificationResearch Use Only
- ClonalityMonoclonal
- Clone IDNOQ7.5.4D
- ConjugateUnconjugated
- Gene ID4625
- Target nameMYH7
- Target descriptionmyosin heavy chain 7
- Target synonymsCMD1S, CMH1, CMYO7A, CMYO7B, CMYP7A, CMYP7B, MPD1, MYHCB, SPMD, SPMM, myosin-7, cardiac muscle myosin heavy chain 7 beta, myHC-beta, myhc-slow, myosin 7, myosin heavy chain beta-subunit, myosin, heavy chain 7, cardiac muscle, beta, myosin, heavy polypeptide 7, cardiac muscle, beta, rhabdomyosarcoma antigen MU-RMS-40.7A
- HostMouse
- IsotypeIgG1
- Protein IDP12883
- Protein NameMyosin-7
- Scientific DescriptionMyosin is a 480 kDa protein known to interact with actin in muscle and in non muscle cells. It contains two identical heavy chains (200 kDa each) and four light chains (15-26 kDa). Myosin molecules consist of two major regions: tail (rod) and heads; they aggregate into filaments through the tail region and interact with actin and with ATP through the head region. Multiple forms of myosin heavy chains exist for each muscle type: skeletal, cardiac, smooth and in non muscle. Myosin isoforms exist in different types of skeletal muscle, depending on the physiological function of the muscle. Mammalian muscle fibers are classified primarily into slow (I), fast-red (IIa) and fast-white (IIb) major types.4,9,10 Changes in the speed of muscle contraction brought about by neural influences result from changes in the pattern of expression of myosin and other myofibrillar genes. Myosin heavy chains are encoded by a multigene family which is developmentally regulated. During differentiation of future muscles, fibers sequentially express heavy chain genes for embryonic, neonatal and adult myosin. Developing muscles express two isoforms of myosin, the embryonic and fetal (neonatal) forms, which are also encoded for by distinct heavy chain genes. The embryonic and fetal myosins have a high calcium-activated ATPase activity at alkaline pH; fibers that contain these myosins are therefore likely to appear similar, histochemically, to mature Type II fibers, even though the latter contain qualitatively distinct mature fast isomyosins. In the neonate, the principal myosin in developing fast and slow muscles is fetal myosin. During the first few weeks of life, fetal myosin is replaced by slow myosin in slow muscle and by fast myosins in fast muscle. There are three adult forms of skeletal myosin corresponding to the three types of muscle fibers. The heavy chains of these myosins are distinct, and are encoded by different heavy chain genes. Muscle fiber type classification for type I and type II fibers has long been applied to the histological study of both normal and pathological human skeletal muscle. Studies of myosin isoforms expression in developing skeletal muscle also provide information about the control of gene expression in different muscle types by neural, hormonal and other factors. The advent of myosin immunocytochemistry, using antibodies to specific myosin heavy chains, has made it possible to trace the developmental history of the complex paths of myosin changes at the cellular level. Antibodies are also important because of the clues they may give concerning the pathogenesis of abnormalities in diseased muscle fibers. Monoclonal antibodies that recognize epitopes peculiar to a particular developmental or mature isomyosin may be used to identify these isomyosins and thus to characterize the temporal pattern of their expression in different fibers during myogenesis.
- ReactivityBovine, Canine, Chicken, Feline, Guinea Pig, Goat, Hamster, Human, Mouse, Porcine, Rabbit, Rat, Sheep
- Storage Instruction-20°C or -80°C,2°C to 8°C
- UNSPSC12352203
References
- Lin CY, Niwa A, Hou CY, et al. Bidirectional myofiber transition through altering the photobiomodulation condition. J Photochem Photobiol B. 2020,212:112041. doi: 10.1016/j.jphotobiol.2020.112041Read this paper
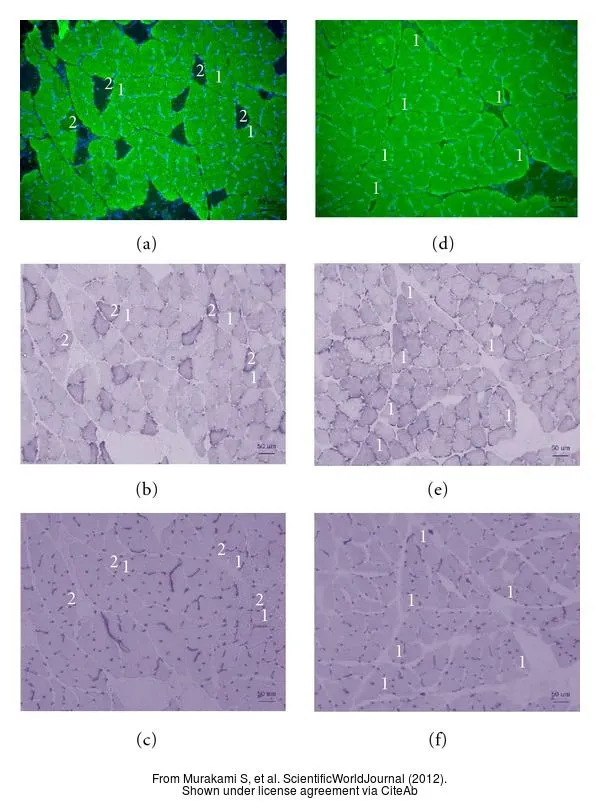
- Murakami S, Fujita N, Kondo H, et al. Abnormalities in the fiber composition and capillary architecture in the soleus muscle of type 2 diabetic Goto-Kakizaki rats. ScientificWorldJournal. 2012,2012:680189. doi: 10.1100/2012/680189Read this paper
- Moreno M, Silvestri E, De Matteis R, et al. 3,5-Diiodo-L-thyronine prevents high-fat-diet-induced insulin resistance in rat skeletal muscle through metabolic and structural adaptations. FASEB J. 2011,25(10):3312-24. doi: 10.1096/fj.11-181982Read this paper
- Hagberg CE, Falkevall A, Wang X, et al. Vascular endothelial growth factor B controls endothelial fatty acid uptake. Nature. 2010,464(7290):917-21. doi: 10.1038/nature08945Read this paper
- Takeda I, Fujino H, Murakami S, et al. Thermal preconditioning prevents fiber type transformation of the unloading induced-atrophied muscle in rats. J Muscle Res Cell Motil. 2009,30(3-4):145-52. doi: 10.1007/s10974-009-9183-zRead this paper

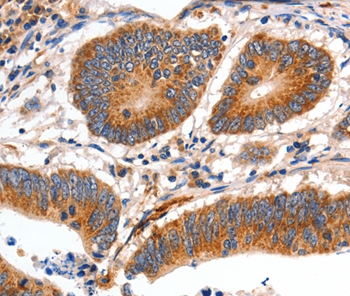
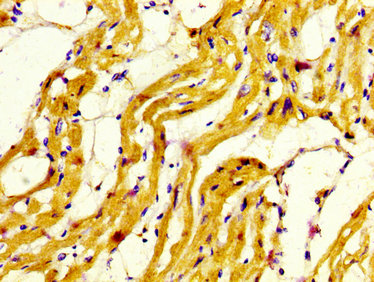
![IHC-P analysis of rabbit tongue tissue using GTX11083 Myosin skeletal slow antibody [NOQ7.5.4D] at 1:4,000. IHC-P analysis of rabbit tongue tissue using GTX11083 Myosin skeletal slow antibody [NOQ7.5.4D] at 1:4,000.](https://www.genetex.com/upload/website/prouct_img/normal/GTX11083/GTX11083_20170605_IHC-P_w_23060500_848.webp)

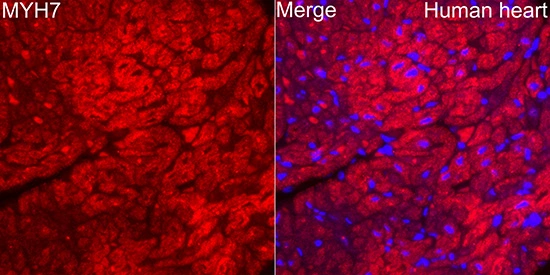
![IHC-P analysis of human heart muscle tissue using GTX04970 MYH7 antibody [MSVA-464M] HistoMAX?. Heart muscle showing a strong cytoplasmic MYH7 staining of all cells.](https://www.genetex.com/upload/website/prouct_img/normal/GTX04970/GTX04970_20241028_IHC-P_2_24102820_391.webp)
![Mouse tissue extract (50 μg) was separated by 5% SDS-PAGE, and the membrane was blotted with MYH7 antibody [N1], N-term (GTX100713) diluted at 1:2000.](https://www.genetex.com/upload/website/prouct_img/normal/GTX100713/GTX100713_40058_20160505_WB_M_muscle_w_23060100_417.webp)