Chemical Structure
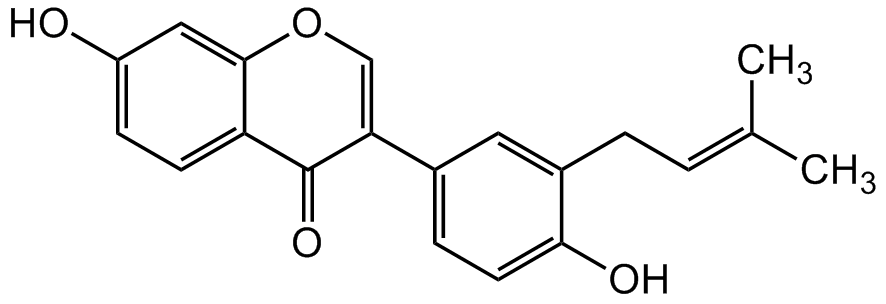
Neobavaisoflavone [41060-15-5] [41060-15-5]
AG-CN2-0498
CAS Number41060-15-5
Product group Chemicals
Estimated Purity>98%
Molecular Weight322.4
Overview
- SupplierAdipoGen Life Sciences
- Product NameNeobavaisoflavone [41060-15-5] [41060-15-5]
- Delivery Days Customer10
- CAS Number41060-15-5
- CertificationResearch Use Only
- Estimated Purity>98%
- Molecular FormulaC20H18O4
- Molecular Weight322.4
- Scientific DescriptionAntibiotic. Antibacterial and antifungal compound. Displays antibiotic activity against Gram-negative multidrug resistant bacteria. Anticancer compound. DNA polymerase inhibitor. Enhances TRAIL-induced apoptosis via inhibition of metastasis. Anti-inflammatory agent inhibiting IL-6-induced STAT3 activation and phosphorylation. Platelet aggregation inhibitor. Human carboxylesterase 1&2 and UDP-glucuronosyltransferase 1A1 inhibitor, enzymes important in drug metabolism. Antioxidant. Inhibits the production of nitric oxide (NO), reactive oxygen species (ROS) and reactive nitrogen species (RNS). Neuroprotective. Exerts protective effects against H2O2-induced neuronal cell damage. Shows osteogenic acitivty. - Chemical. CAS: 41060-15-5. Formula: C20H18O4. MW: 322.4. Isolated from Psoralea corylifolia sp. Antibiotic. Antibacterial and antifungal compound. Displays antibiotic activity against Gram-negative multidrug resistant bacteria. Anticancer compound. DNA polymerase inhibitor. Enhances TRAIL-induced apoptosis via inhibition of metastasis. Anti-inflammatory agent inhibiting IL-6-induced STAT3 activation and phosphorylation. Platelet aggregation inhibitor. Human carboxylesterase 1&2 and UDP-glucuronosyltransferase 1A1 inhibitor, enzymes important in drug metabolism. Antioxidant. Inhibits the production of nitric oxide (NO), reactive oxygen species (ROS) and reactive nitrogen species (RNS). Neuroprotective. Exerts protective effects against H2O2-induced neuronal cell damage. Shows osteogenic acitivty.
- SMILESOC1=CC=C(C(C(C2=CC=C(O)C(C/C=C(C)/C)=C2)=CO3)=O)C3=C1
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200