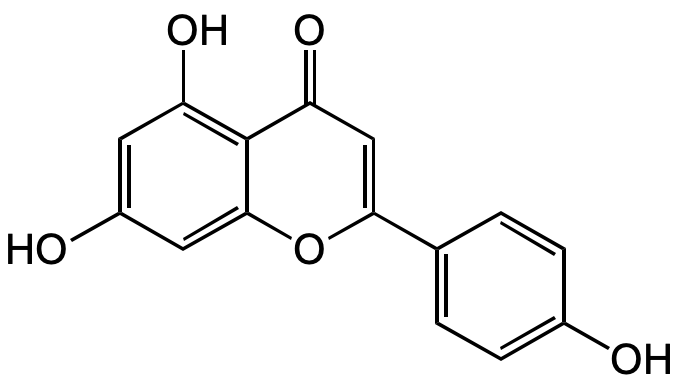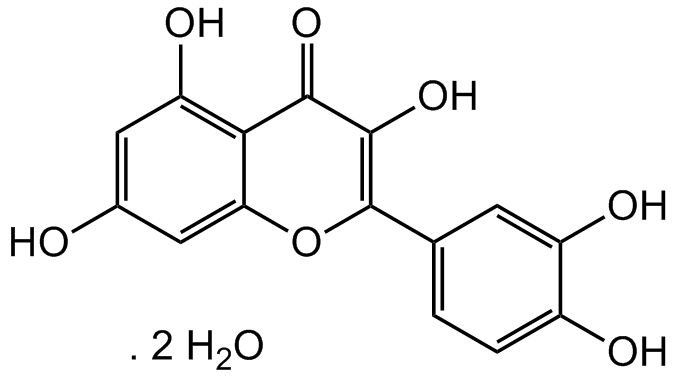
Chemical Structure
Quercetin . dihydrate [6151-25-3] [6151-25-3]
AG-CN2-0409
CAS Number6151-25-3
Product group Chemicals
Estimated Purity>95%
Molecular Weight302.2 . 36.0
Overview
- SupplierAdipoGen Life Sciences
- Product NameQuercetin . dihydrate [6151-25-3] [6151-25-3]
- Delivery Days Customer10
- CAS Number6151-25-3
- CertificationResearch Use Only
- Estimated Purity>95%
- Hazard InformationDanger,Excepted quantity
- Molecular FormulaC15H10O7 . 2H20
- Molecular Weight302.2 . 36.0
- Scientific DescriptionChemical. CAS: 6151-25-3. Formula: C15H10O7 . 2H20. MW: 302.2 . 36.0. Isolated from Umbelliferae sp. Multipotent flavonoid antioxidant. Potent free radical scavenger. Neuroprotective. Anticancer compound with chemosensitizing activity. Cell cycle arrest, apoptosis and autophagy inducer. Proteasome inhibitor. Pleiotropic kinase inhibitor, including tyrosine protein kinase (Trk), mitochondrial ATPase, cAMP- and cGMP-phosphodiesterases, PI3-kinase activity, phospholipase A2 and protein kinase C (PKC). DNA topoisomerases inhibitor. SIRT1 activator. Heat shock proteins inhibitor. Reversible fatty acid synthase inhibitor. Antithrombotic, antihistaminic and anti-inflammatory agent. Monoamine oxidase inhibitor. Vasodilatory compound. anti-diabetic compound. - Multipotent flavonoid antioxidant. Potent free radical scavenger. Neuroprotective. Anticancer compound with chemosensitizing activity. Cell cycle arrest, apoptosis and autophagy inducer. Proteasome inhibitor. Pleiotropic kinase inhibitor, including tyrosine protein kinase (Trk), mitochondrial ATPase, cAMP- and cGMP-phosphodiesterases, PI3-kinase activity, phospholipase A2 and protein kinase C (PKC). DNA topoisomerases inhibitor. SIRT1 activator. Heat shock proteins inhibitor. Reversible fatty acid synthase inhibitor. Antithrombotic, antihistaminic and anti-inflammatory agent. Monoamine oxidase inhibitor. Vasodilatory compound. Anti-diabetic compound. Antiviral for the prevention and treatment of SARS-CoV-2 related disease (COVID-19)
- SMILESOC1=CC(O)=C2C(OC(=C(O)C2=O)C2=CC=C(O)C(O)=C2)=C1
- Storage Instruction2°C to 8°C
- UN NumberUN 2811
- UNSPSC12352200