Sheep anti Human chx 10 (Visual system homeobox 2) (CT)
X1179P
ApplicationsWestern Blot, ImmunoHistoChemistry, ImmunoHistoChemistry Frozen
Product group Antibodies
ReactivityChicken, Human, Mouse, Rat
TargetVSX2
Overview
- SupplierNordic-MUbio
- Product NameSheep anti Human chx 10 (Visual system homeobox 2) (CT)
- Delivery Days Customer7
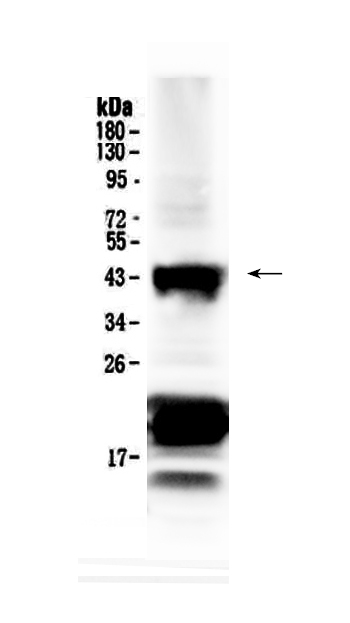
- Application Supplier NoteDetects Chx10 by Western blot at 0.5 to 1 microg/ml. Detects a 46 kDa band in mouse and rat retinal tissue lysates. Optimal concentration should be evaluated by serial dilutions. Suitable for use against recombinant proteins conjugated to OVA, GST, His tags and other.
- ApplicationsWestern Blot, ImmunoHistoChemistry, ImmunoHistoChemistry Frozen
- Applications SupplierWestern Blotting;Immunohistochemistry (frozen)
- CertificationResearch Use Only
- ClonalityPolyclonal
- ConjugateUnconjugated
- Gene ID338917
- Target nameVSX2
- Target descriptionvisual system homeobox 2
- Target synonymsCHX10, HOX10, MCOP2, MCOPCB3, RET1, visual system homeobox 2, ceh-10 homeo domain containing homolog, ceh-10 homeodomain-containing homolog, homeobox protein CHX10
- HostSheep
- IsotypeIgG
- Protein IDP58304
- Protein NameVisual system homeobox 2
- Scientific DescriptionCeh-10 homeodomain-containing homolog
- Shelf life instructionSee expiration date on vial
- ReactivityChicken, Human, Mouse, Rat
- Reactivity SupplierHuman;Mouse;Rat;Chicken
- UNSPSC12352203