Chemical Structure
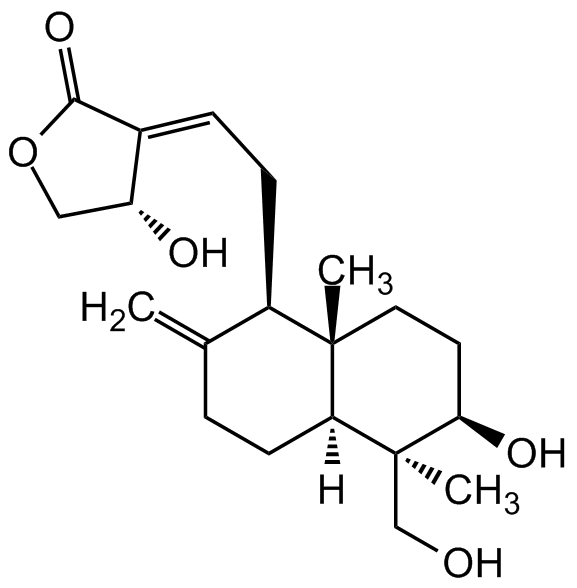
Andrographolide [5508-58-7] [5508-58-7]
CDX-A0576
CAS Number5508-58-7
Product group Chemicals
Estimated Purity>98%
Molecular Weight350.455
Overview
- SupplierChemodex
- Product NameAndrographolide [5508-58-7] [5508-58-7]
- Delivery Days Customer2
- CAS Number5508-58-7
- CertificationResearch Use Only
- Estimated Purity>98%
- Hazard InformationWarning
- Molecular FormulaC20H30O5
- Molecular Weight350.455
- Scientific DescriptionAndrographolide, a bioactive diterpene lactone, is the main constituent of Andrographis paniculata, a plant used in traditional medicines. It is known to have different biological properties such as anti-inflammatory, immunosuppressive, antidiabetic, antihyperglycemic, antibacterial, antioxidant, antinoniceptive, antimalarial, anticancer and hepatoprotective activity. It also shows potent anti-viral effect against dengue virus. It blocks T-cell proliferation and the proliferation of several cancer cell lines in vitro. It acts as an irreversible antagonist of NF-kappa and AP-1 (IC50 < 15 microM) activation, and prevents in vitro and in vivo T cell activation. - Chemical. CAS: 5508-58-7. Formula: C20H30O5. MW: 350.455. Andrographolide, a bioactive diterpene lactone, is the main constituent of Andrographis paniculata, a plant used in traditional medicines. It is known to have different biological properties such as anti-inflammatory, immunosuppressive, antidiabetic, antihyperglycemic, antibacterial, antioxidant, antinoniceptive, antimalarial, anticancer and hepatoprotective activity. It also shows potent anti-viral effect against dengue virus. It blocks T-cell proliferation and the proliferation of several cancer cell lines in vitro. It acts as an irreversible antagonist of NF-kappa and AP-1 (IC50 < 15 microM) activation, and prevents in vitro and in vivo T cell activation.
- SMILESC=C1CC[C@@]([C@@](CO)(C)[C@H](O)CC2)([H])[C@]2(C)[C@@H]1C/C=C3C(OC[C@H]/3O)=O
- Storage Instruction-20°C
- UNSPSC12352200