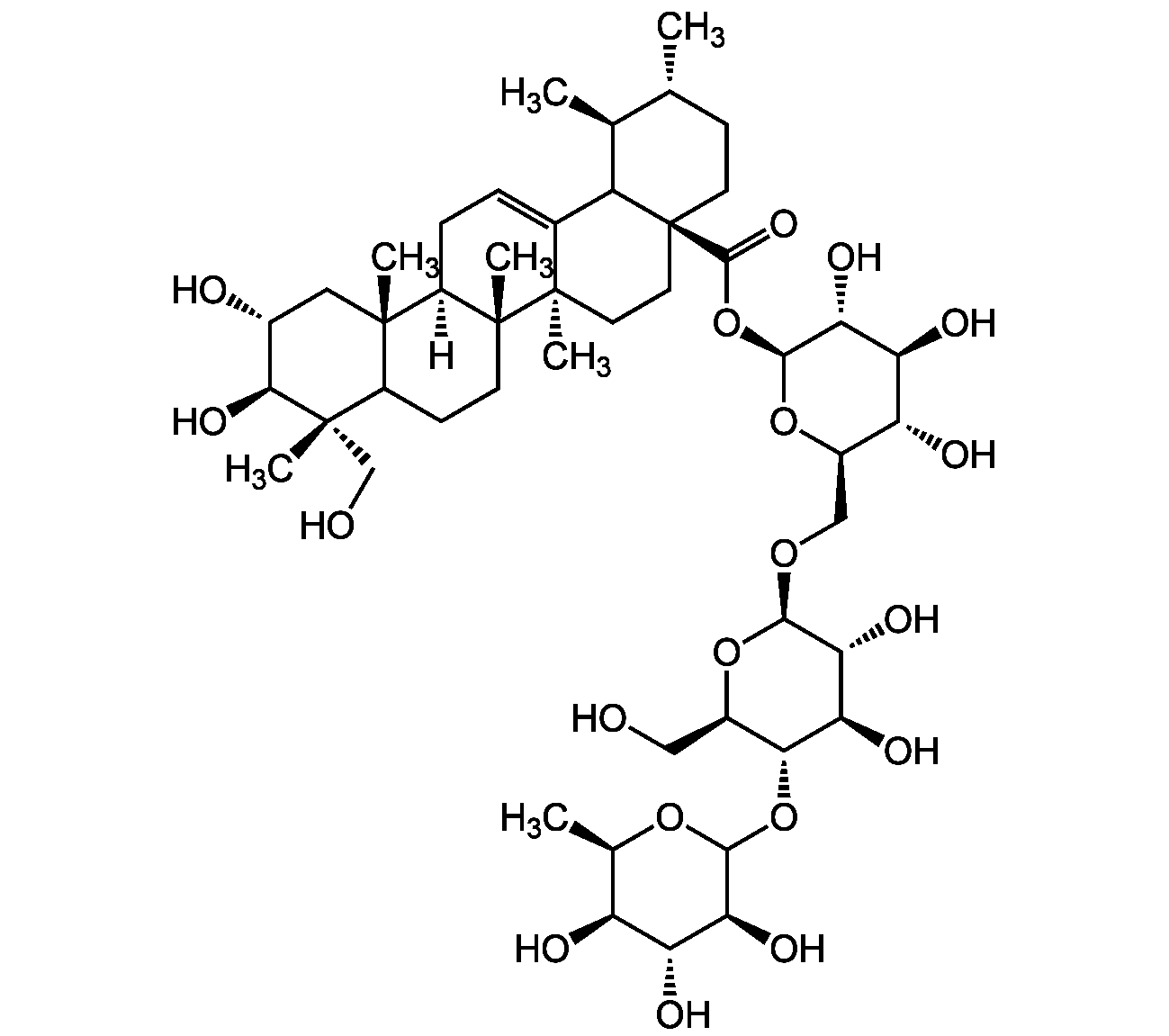
Chemical Structure
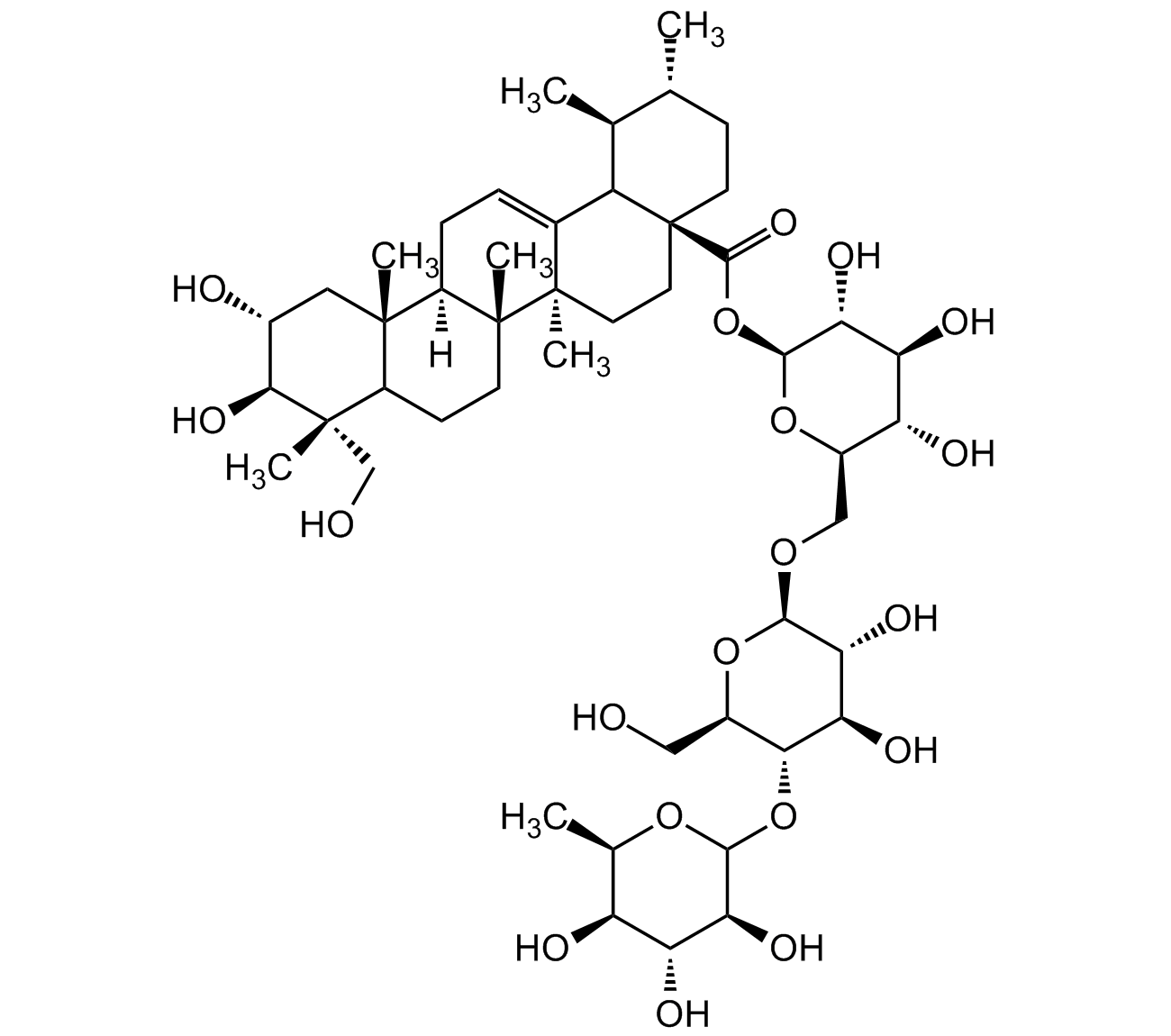
Asiaticoside [16830-15-2] [16830-15-2]
CDX-A0575
CAS Number16830-15-2
Product group Chemicals
Estimated Purity>95%
Molecular Weight959.133
Overview
- SupplierChemodex
- Product NameAsiaticoside [16830-15-2] [16830-15-2]
- Delivery Days Customer2
- CAS Number16830-15-2
- CertificationResearch Use Only
- Estimated Purity>95%
- Molecular FormulaC48H78O19
- Molecular Weight959.133
- Scientific DescriptionAsiaticoside is the main saponin constituent of C. asiatica, a plant long used in the Ayurvedic system of medicine to treat dermatitis, diabetes, cough, cataract, hypertension, as well as for wound healing and improving memory. It is a human collagen I synthesis inducer with anti-wrinkle activity. It shows wound healing activity, enhancing normal human skin cell migration, attachment and growth. Asiaticoside is an anti-inflammatory compound that inibits MAPKs, NF-kappa, COX-2 and iNOS expression, and production of TNF-alpha and IL-6. Also shown to induce apoptosis and autophagy in cancer cells. Described to have also antibacterial, antioxidant, anxiolytic, antidepressant-like, hepatoprotective, neuroprotective and antileishmanial activity. - Chemical. CAS: 16830-15-2. Formula: C48H78O19. MW: 959.133. Asiaticoside is the main saponin constituent of C. asiatica, a plant long used in the Ayurvedic system of medicine to treat dermatitis, diabetes, cough, cataract, hypertension, as well as for wound healing and improving memory. It is a human collagen I synthesis inducer with anti-wrinkle activity. It shows wound healing activity, enhancing normal human skin cell migration, attachment and growth. Asiaticoside is an anti-inflammatory compound that inibits MAPKs, NF-kappa, COX-2 and iNOS expression, and production of TNF-alpha and IL-6. Also shown to induce apoptosis and autophagy in cancer cells. Described to have also antibacterial, antioxidant, anxiolytic, antidepressant-like, hepatoprotective, neuroprotective and antileishmanial activity.
- SMILESO[C@H]1[C@H](O)[C@@](C)(CO)C(CC[C@]2(C)[C@]3([H])CC=C4[C@@]2(C)CC[C@]5(C(O[C@@H]6O[C@H](CO[C@@H]7O[C@H](CO)[C@@H](OC8O[C@H](C)[C@H](O)[C@@H](O)[C@@H]8O)[C@H](O)[C@H]7O)[C@@H](O)[C@H](O)[C@H]6O)=O)C4[C@@H](C)[C@H](C)CC5)[C@]3(C)C1
- Storage Instruction2°C to 8°C
- UNSPSC12352200


![Asiaticoside [16830-15-2]](https://www.targetmol.com/group3/M00/03/44/CgoaEWY7RnGEFodjAAAAAG-RD-w684.png)