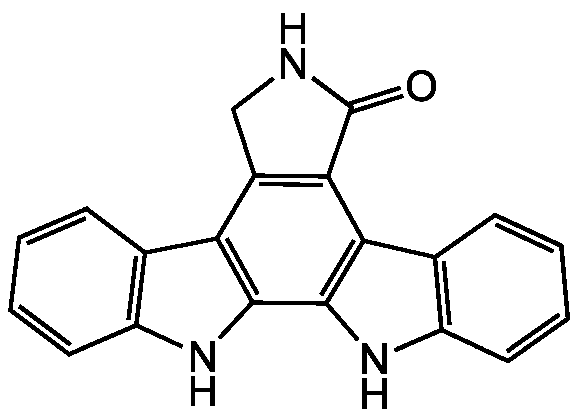
Chemical Structure
K-252c [85753-43-1] [85753-43-1]
AG-CN2-0097
CAS Number85753-43-1
Product group Chemicals
Estimated Purity>97%
Molecular Weight311.4
Overview
- SupplierAdipoGen Life Sciences
- Product NameK-252c [85753-43-1] [85753-43-1]
- Delivery Days Customer10
- CAS Number85753-43-1
- CertificationResearch Use Only
- Estimated Purity>97%
- Molecular FormulaC20H13N3O
- Molecular Weight311.4
- Scientific DescriptionChemical. CAS: 85753-43-1. Formula: C20H13N3O. MW: 311.4. Isolated from Streptomyces longisporoflavus. Indolocarbazole alkaloid antibiotic. Potent cell permeable reversible and ATP-competitive PKC (protein kinase C) inhibitor with 10-fold selectivity over PKA (protein kinase A). Anticancer compound. Cytotoxic againt various cancer cell lines. Apoptosis inducer. Antiviral compound against GCV-sensitive and -resistant strains of human cytomegalovirus (HCMV). LACTB (beta-lactamase), malate dehydrogenase (MDH2, MDHC, MDH1B, ME1. ME2, ME3) and chymotrypsin inhibitor. Inhibits mixed lineage kinase (MLK). - Indolocarbazole alkaloid antibiotic. [1]. Potent cell permeable reversible and ATP-competitive PKC (protein kinase C) inhibitor with 10-fold selectivity over PKA (protein kinase A) [1-5]. Anticancer compound. Cytotoxic againt various cancer cell lines [6, 9,11]. Apoptosis inducer [11]. Antiviral compound against GCV-sensitive and -resistant strains of human cytomegalovirus (HCMV) [7]. LACTB (beta-lactamase), malate dehydrogenase (MDH2, MDHC, MDH1B, ME1. ME2, ME3) and chymotrypsin inhibitor [8]. Inhibits mixed lineage kinase (MLK) [10].
- SMILESO=C1NCC2=C1C1=C(NC3=C1C=CC=C3)C1=C2C2=C(N1)C=CC=C2
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200

![K-252c [85753-43-1] [85753-43-1]](https://www.targetmol.com/group3/M00/36/AE/CgoaEGayQi6EDjorAAAAAMeZBHs717.png)