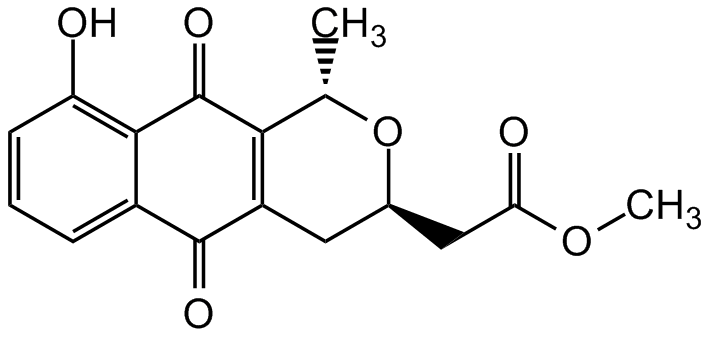
Chemical Structure
OM173-alphaA [58286-56-9] [58286-56-9]
AG-CN2-0158
CAS Number58286-56-9
Product group Chemicals
Estimated Purity>95%
Molecular Weight316.3
Overview
- SupplierAdipoGen Life Sciences
- Product NameOM173-alphaA [58286-56-9] [58286-56-9]
- Delivery Days Customer10
- CAS Number58286-56-9
- CertificationResearch Use Only
- Estimated Purity>95%
- Hazard InformationWarning
- Molecular FormulaC17H16O6
- Molecular Weight316.3
- Scientific DescriptionAntibiotic [1-3]. Antimycoplasma [1, 3, 4]. Antifungal compound [1, 3]. Anti-malarial agent [5]. Ras-competitive non-CAAX mimetic type farnesyltransferase (FTase) inhibitor [6]. Potential anticancer compound. Inducer of antiproliferative effects in tumor cell lines [6-8]. Selective DNA (cytosine-5)-methyltransferase 3B (DNMT3B) inhibitor [8]. - Chemical. CAS: 58286-56-9. Formula: C17H16O6. MW: 316.3. Isolated from Streptomyces sp. Antibiotic. Antimycoplasma. Antifungal compound. Anti-malarial agent. Ras-competitive non-CAAX mimetic type farnesyltransferase (FTase) inhibitor. Potential anticancer compound. Inducer of antiproliferative effects in tumor cell lines. Selective DNA (cytosine-5)-methyltransferase 3B (DNMT3B) inhibitor.
- SMILESCOC(=O)C[C@H]1CC2=C([C@H](C)O1)C(=O)C1=C(O)C=CC=C1C2=O
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200

![OM173-alphaA [58286-56-9] [58286-56-9]](https://www.targetmol.com/group3/M00/03/35/CgoaEWY7RJ2ELnHdAAAAAG5L8gA894.png)