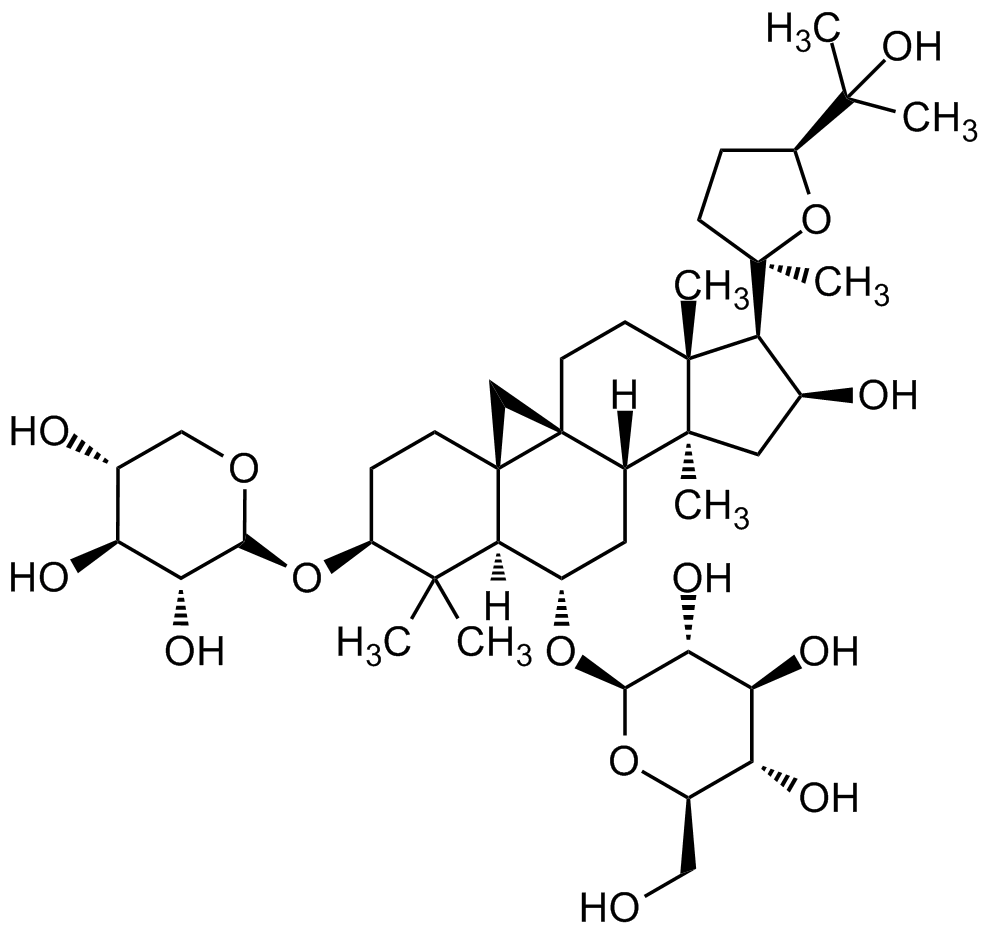
Chemical Structure
Astragaloside IV [84687-43-4] [84687-43-4]
CDX-A0577
CAS Number84687-43-4
Product group Chemicals
Estimated Purity>98%
Molecular Weight784.98
Overview
- SupplierChemodex
- Product NameAstragaloside IV [84687-43-4] [84687-43-4]
- Delivery Days Customer2
- CAS Number84687-43-4
- CertificationResearch Use Only
- Estimated Purity>98%
- Molecular FormulaC41H68O14
- Molecular Weight784.98
- Scientific DescriptionAstragaloside IV (AS-IV) is a bioactive saponin and constituent of the traditional chinese medicine plant Astragali radix. AS-IV has multiple pharmacologic effects, including anti-inflammatory, antioxidative, anti-asthma, antidiabetes, anticancer, immunoregulation, neuroprotective and cardioprotective properties via numerous signaling pathways. This includes inhibition of NF-kappaB, inducing hypoxia-inducible factor-1alpha accumulation via PI3K/Akt pathway, reducing Abeta production in Alzheimers disease through inhibition of BACE1, working as a natural PPARgamma agonist, improving lipid metabolism in obese mice, enhancing chemosensitivity through CD276 (B7-H3)-pathway inhibition, and suppressing glucose-induced NLRP3 inflammasome activation. - Chemical. CAS: 84687-43-4. Formula: C41H68O14. MW: 784.98. Astragaloside IV (AS-IV) is a bioactive saponin and constituent of the traditional chinese medicine plant Astragali radix. AS-IV has multiple pharmacologic effects, including anti-inflammatory, antioxidative, anti-asthma, antidiabetes, anticancer, immunoregulation, neuroprotective and cardioprotective properties via numerous signaling pathways. This includes inhibition of NF-kappaB, inducing hypoxia-inducible factor-1alpha accumulation via PI3K/Akt pathway, reducing Abeta production in Alzheimers disease through inhibition of BACE1, working as a natural PPARgamma agonist, improving lipid metabolism in obese mice, enhancing chemosensitivity through B7-H3 inhibition, and suppressing glucose-induced NLRP3 inflammasome activation.
- SMILESO[C@H]1C[C@@]2(C)[C@]3([H])C[C@H](O[C@@H]4O[C@H](CO)[C@@H](O)[C@H](O)[C@H]4O)[C@@]5([H])C(C)(C)[C@@H](O[C@H]6OC[C@@H](O)[C@H](O)[C@H]6O)CC[C@]57[C@]3(C7)CC[C@]2(C)[C@H]1[C@]8(C)O[C@H](C(C)(O)C)CC8
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200